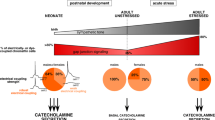
Abstract
The current view of stimulation-secretion coupling in adrenal neuroendocrine chromaffin cells holds that catecholamines are released upon transsynaptic sympathetic stimulation mediated by acetylcholine released from the splanchnic nerve terminals. However, this traditional vertical scheme would merit to be revisited in the light of recent data. Although electrical discharges invading the splanchnic nerve endings are the major physiological stimulus to trigger catecholamine release in vivo, growing evidence indicates that intercellular chromaffin cell communication mediated by gap junctions represents an additional route by which biological signals (electrical activity, changes in intracellular Ca2+ concentration,…) propagate between adjacent cells and trigger subsequent catecholamine exocytosis. Accordingly, it has been proposed that gap junctional communication efficiently helps synapses to lead chromaffin cell function and, in particular, hormone secretion. The experimental clues supporting this hypothesis are presented and discussed with regards to both interaction with the excitatory cholinergic synaptic transmission and physiopathology of the adrenal medulla.




Similar content being viewed by others
References
Axelrod J (1971) Noradrenaline: fate and control of its synthesis. Science 173:598–606
de Diego AM, Gandía L, García AG (2008) A physiological view of the central and peripheral mechanisms that regulate the release of catecholamines at the adrenal medulla. Acta Physiol 192:287–301
Dreyer GP (1898) On secretory nerves to the suprarenal capsules. Am J Physiol 2:203–219
Feldberg W, Minz B, Tsudzimura H (1934) The mechanism of the nervous discharge of adrenaline. J Physiol 81:286–304
Elliott TR (1912) The control of the suprarenal glands by the splanchnic nerves. J Physiol 44:374–409
Elliott TR (1913) The innervation of the adrenal glands. J Physiol 46:285–290
Hillarp NA (1946) Functional organization of the peripheral autonomic innervation. Acta Anat 4(Suppl):1–153
Coupland RE (1965) Electron microscopic observations on the structure of the rat adrenal medulla. II. Normal innervation. J Anat 99:255–272
Carmichael SW (1986) Morphology and innervation of the adrenal medulla. In: Rosenheek K, Lelkes P (eds) Stimulus-secretion coupling, vol 1. CRC, Boca Raton, FL, pp 1–29
Coupland RE (1989) The natural history of the chromaffin cell—twenty-five years on the beginning. Arch Histol Cytol 52:331–341
Iijima T, Matsumoto G, Kidokoro Y (1992) Synaptic activation of rat adrenal medulla examined with a large photodiode array in combination with a voltage-sensitive dye. Neuroscience 51:211–219
Unwin PN (1987) Gap junction structure and the control of cell-to-cell communication. Ciba Found Symp 125:78–91
Sáez JC, Berthoud VM, Branes MC, Martinez AD, Beyer EC (2003) Plasma membrane channels formed by connexins: their regulation and functions. Physiol Rev 83:1359–1400
Mehta PP (2007) Introduction: a tribute to cell-to-cell channels. J Membr Biol 217:5–12
Bruzzone R, Hormuzdi SG, Barbe MT, Herb A, Monyer H (2003) Pannexins, a family of gap junction proteins expressed in brain. Proc Natl Acad Sci U S A 100:13644–13649
Panchin YV (2005) Evolution of gap junction proteins—the pannexin alternative. J Exp Biol 208:1415–1419
Barbe MT, Monyer H, Bruzzone R (2006) Cell–cell communication beyond connexins: the pannexin channels. Physiology (Bethesda) 21:103–114
Shestopalov VI, Panchin Y (2008) Pannexins and gap junction protein diversity. Cell Mol Life Sci 65:376–394
Murray SA, Pharrams SY (1997) Comparison of gap junction expression in the adrenal gland. Microsc Res Tech 36:510–519
Grynszpan-Wynograd O, Nicolas G (1980) Intercellular junctions in the adrenal medulla: a comparative freeze-fracture study. Tissue Cell 12:661–672
Meda P, Pepper MS, Traub O, Willecke K, Gros D, Beyer E, Nicholson B, Paul D, Orci L (1993) Differential expression of gap junction connexins in endocrine and exocrine glands. Endocrinology 133:2371–2378
Martin AO, Mathieu M-N, Chevillard C, Guérineau NC (2001) Gap junctions mediate electrical signaling and ensuing cytosolic Ca2+ increases between chromaffin cells in adrenal slices: a role in catecholamine release. J Neurosci 21:5397–5405
Colomer C, Olivos Ore LA, Coutry N, Mathieu M-N, Arthaud S, Fontanaud P, Iankova I, Macari F, Thouënnon E, Yon L, Anouar Y, Guérineau NC (2008a) Functional remodeling of gap junction-mediated electrical communication between adrenal chromaffin cells in stressed rats. J Neurosci 28:6616–6626
Willenberg HS, Schott M, Saeger W, Tries A, Scherbaum WA, Bornstein SR (2006) Expression of connexins in chromaffin cells of normal human adrenals and in benign and malignant pheochromocytomas. Ann N Y Acad Sci 1073:578–583
Li X, Olson C, Lu S, Nagy JI (2004) Association of connexin36 with zonula occludens-1 in HeLa cells, betaTC-3 cells, pancreas, and adrenal gland. Histochem Cell Biol 122:485–498
Degen J, Meier C, Van Der Giessen RS, Söhl G, Petrasch-Parwez E, Urschel S, Dermietzel R, Schilling K, De Zeeuw CI, Willecke K (2004) Expression pattern of lacZ reporter gene representing connexin36 in transgenic mice. J Comp Neurol 473:511–525
Eiberger J, Kibschull M, Strenzke N, Schober A, Büssow H, Wessig C, Djahed S, Reucher H, Koch DA, Lautermann J, Moser T, Winterhager E, Willecke K (2006) Expression pattern and functional characterization of connexin29 in transgenic mice. Glia 53:601–611
Condorelli DF, Belluardo N, Trovato-Salinaro A, Mudò G (2000) Expression of Cx36 in mammalian neurons. Brain Res Brain Res Rev 32:72–85
Lu SJ, Li H, Zhou FH, Zhang JJ, Wang LX (2007) Connexin 36 is expressed and associated with zonula occludens-1 protein in PC-12 cells. Gen Physiol Biophys 26:33–39
Falk MM (2000) Biosynthesis and structural composition of gap junction intercellular membrane channels. Eur J Cell Biol 79:564–574
Dermietzel R, Meier C, Bukauskas F, Spray DC (2003) Following tracks of hemichannels. Cell Commun Adhes 10:335–340
Malchow RP, Qian H, Ripps H (1993) Evidence for hemi-gap junctional channels in isolated horizontal cells of the skate retina. J Neurosci Res 35:237–245
Hofer A, Dermietzel R (1998) Visualization and functional blocking of gap junction hemichannels (connexons) with antibodies against external loop domains in astrocytes. Glia 24:141–154
Ebihara L, Xu X, Oberti C, Beyer EC, Berthoud VM (1999) Co-expression of lens fiber connexins modifies hemi-gap-junctional channel behavior. Biophys J 76:198–206
Kondo RP, Wang SY, John SA, Weiss JN, Goldhaber JI (2000) Metabolic inhibition activates a non-selective current through connexin hemichannels in isolated ventricular myocytes. J Mol Cell Cardiol 32:1859–1872
Belliveau DJ, Bani-Yaghoub M, McGirr B, Naus CC, Rushlow WJ (2006) Enhanced neurite outgrowth in PC-12 cells mediated by connexin hemichannels and ATP. J Biol Chem 281:20920–20931
Bennett MV, Contreras JE, Bukauskas FF, Sáez JC (2003) New roles for astrocytes: gap junction hemichannels have something to communicate. Trends Neurosci 26:610–617
Sáez JC, Contreras JE, Bukauskas FF, Retamal MA, Bennett MV (2003) Gap junction hemichannels in astrocytes of the CNS. Acta Physiol Scand 179:9–22
Kamermans M, Fahrenfort I (2004) Ephaptic interactions within a chemical synapse: hemichannel-mediated ephaptic inhibition in the retina. Curr Opin Neurobiol 14:531–541
Trexler EB, Bennett MV, Bargiello TA, Verselis VK (1996) Voltage gating and permeation in a gap junction hemichannel. Proc Natl Acad Sci U S A 93:5836–5841
Evans WH, De Vuyst E, Leybaert L (2006) The gap junction cellular internet: connexin hemichannels enter the signalling limelight. Biochem J 397:1–14
Spray DC, Ye ZC, Ransom BR (2006) Functional connexin “hemichannels”: a critical appraisal. Glia 54:758–773
Schock SC, Leblanc D, Hakim AM, Thompson CS (2008) ATP release by way of connexin 36 hemichannels mediates ischemic tolerance in vitro. Biochem Biophys Res Commun 368:138–144
Kang J, Kang N, Lovatt D, Torres A, Zhao Z, Lin J, Nedergaard M (2008) Connexin 43 hemichannels are permeable to ATP. J Neurosci 28:4702–4711
Takeuchi H, Jin S, Wang J, Zhang G, Kawanokuchi J, Kuno R, Sonobe Y, Mizuno T, Suzumura A (2006) Tumor necrosis factor-alpha induces neurotoxicity via glutamate release from hemichannels of activated microglia in an autocrine manner. J Biol Chem 281:21362–21368
Retamal MA, Froger N, Palacios-Prado N, Ezan P, Sáez PJ, Sáez JC, Giaume C (2007) Cx43 hemichannels and gap junction channels in astrocytes are regulated oppositely by proinflammatory cytokines released from activated microglia. J Neurosci 27:13781–13792
De Vuyst E, Decrock E, De Bock M, Yamasaki H, Naus CC, Evans WH, Leybaert L (2007) Connexin hemichannels and gap junction channels are differentially influenced by lipopolysaccharide and basic fibroblast growth factor. Mol Biol Cell 18:34–46
Nassar-Gentina V, Pollard HB, Rojas E (1988) Electrical activity in chromaffin cells of intact mouse adrenal gland. Am J Physiol 254:C675–C683
Holman ME, Coleman HA, Tonta MA, Parkington HC (1994) Synaptic transmission from splanchnic nerves to the adrenal medulla of guinea-pigs. J Physiol 478:115–124
Moser T (1998) Low-conductance intercellular coupling between mouse chromaffin cells in situ. J Physiol 506:195–205
Martin AO, Mathieu M-N, Guérineau NC (2003) Evidence for long-lasting cholinergic control of gap junctional communication between adrenal chromaffin cells. J Neurosci 23:3669–3678
Martin AO, Alonso G, Guérineau NC (2005) Agrin mediates a rapid switch from electrical coupling to chemical neurotransmission during synaptogenesis. J Cell Biol 169:503–514
Hillarp NA, Hokfelt B (1953) Evidence of adrenaline and noradrenaline in separate adrenal medullary cells. Acta Physiol Scand 30:55–68
Grynszpan-Winograd O (1974) Adrenaline and noradrenaline cells in the adrenal medulla of the hamster: a morphological study of their innervation. J Neurocytol 3:341–361
Bereiter DA, Engeland WC, Gann DS (1987) Adrenal secretion of epinephrine after stimulation of trigeminal nucleus caudalis depends on stimulus pattern. Neuroendocrinology 45:54–61
Edwards SL, Anderson CR, Southwell BR, McAllen RM (1996) Distinct preganglionic neurons innervate noradrenaline and adrenaline cells in the cat adrenal medulla. Neuroscience 70:825–832
Aunis D, Langley K (1999) Physiological aspects of exocytosis in chromaffin cells of the adrenal medulla. Acta Physiol Scand 167:89–97
Kameda Y (1996) Immunoelectron microscopic localization of vimentin in sustentacular cells of the carotid body and the adrenal medulla of guinea pigs. J Histochem Cytochem 44:1439–1449
Díaz-Flores L, Gutiérrez R, Varela H, Valladares F, Alvarez-Argüelles H, Borges R (2008) Histogenesis and morphofunctional characteristics of chromaffin cells. Acta Physiol 192:145–163
Orthmann-Murphy JL, Abrams CK, Scherer SS (2008) Gap junctions couple astrocytes and oligodendrocytes. J Mol Neurosci 35:101–116
Morand I, Fonlupt P, Guerrier A, Trouillas J, Calle A, Remy C, Rousset B, Munari-Silem Y (1996) Cell-to-cell communication in the anterior pituitary: evidence for gap junction-mediated exchanges between endocrine cells and folliculostellate cells. Endocrinology 137:3356–3367
Fauquier T, Guérineau NC, McKinney RA, Bauer K, Mollard P (2001) Folliculostellate cell network: a route for long-distance communication in the anterior pituitary. Proc Natl Acad Sci U S A 98:8891–8896
Anderson DJ (1993) Molecular control of cell fate in the neural crest: the sympathoadrenal lineage. Annu Rev Neurosci 16:129–158
Huber K (2006) The sympathoadrenal cell lineage: specification, diversification, and new perspectives. Dev Biol 298:335–343
Ceña V, Nicolas GP, Sanchez-Garcia P, Kirpekar SM, Garcia AG (1983) Pharmacological dissection of receptor-associated and voltage-sensitive ionic channels involved in catecholamine release. Neuroscience 10:1455–1462
Acheson AL, Thoenen H (1983) Cell contact-mediated regulation of tyrosine hydroxylase synthesis in cultured bovine adrenal chromaffin cells. J Cell Biol 97:925–928
Saadat S, Thoenen H (1986) Selective induction of tyrosine hydroxylase by cell–cell contact in bovine adrenal chromaffin cells is mimicked by plasma membranes. J Cell Biol 103:1991–1997
Saadat S, Stehle AD, Lamouroux A, Mallet J, Thoenen H (1987) Influence of cell–cell contact on levels of tyrosine hydroxylase in cultured bovine adrenal chromaffin cells. J Biol Chem 262:13007–13014
Kajiwara R, Sand O, Kidokoro Y, Barish ME, Iijima T (1997) Functional organization of chromaffin cells and cholinergic synaptic transmission in rat adrenal medulla. Jpn J Physiol 47:449–464
Weinstock M, Razin M, Schorer-Apelbaum D, Men D, McCarty R (1998) Gender differences in sympathoadrenal activity in rats at rest and in response to footshock stress. Int J Dev Neurosci 16:289–295
Fernández-Ruiz JJ, Bukhari AR, Martínez-Arrieta R, Tresguerres JA, Ramos JA (1988) Effects of estrogens and progesterone on the catecholaminergic activity of the adrenal medulla in female rats. Life Sci 42:1019–1028
Fernandez-Ruiz JJ, Bukhari AR, Hernandez ML, Alemany J, Ramos JA (1989) Sex- and age-related changes in catecholamine metabolism and release of rat adrenal gland. Neurobiol Aging 10:331–335
Valiunas V, Bukauskas FF, Weingart R (1997) Conductances and selective permeability of connexin43 gap junction channels examined in neonatal rat heart cells. Circ Res 80:708–719
Srinivas M, Rozental R, Kojima T, Dermietzel R, Mehler M, Condorelli DF, Kessler JA, Spray DC (1999) Functional properties of channels formed by the neuronal gap junction protein connexin36. J Neurosci 19:9848–9855
Petrocelli T, Lye SJ (1993) Regulation of transcripts encoding the myometrial gap junction protein, connexin-43, by estrogen and progesterone. Endocrinology 133:284–290
Yu W, Dahl G, Werner R (1994) The connexin43 gene is responsive to oestrogen. Proc Biol Sci 255:125–132
Shinohara K, Funabashi T, Nakamura TJ, Kimura F (2001) Effects of estrogen and progesterone on the expression of connexin-36 mRNA in the suprachiasmatic nucleus of female rats. Neurosci Lett 309:37–40
Gulinello M, Etgen AM (2005) Sexually dimorphic hormonal regulation of the gap junction protein, CX43, in rats and altered female reproductive function in CX43+/− mice. Brain Res 1045:107–115
Gonzalez D, Gomez-Hernandez JM, Barrio LC (2007) Molecular basis of voltage dependence of connexin channels: an integrative appraisal. Prog Biophys Mol Biol 94:66–106
Yamagami K, Moritoyo T, Wakamori M, Sorimachi M (2002) Limited intercellular spread of spontaneous Ca2+ signals via gap junctions between mouse chromaffin cells in situ. Neurosci Lett 323:97–100
Martin AO, Alonso G, Guérineau NC (2005) An unexpected role for agrin on cell-to-cell coupling during synaptogenesis. Med Sci (Paris) 21:913–915
Metz-Boutigue MH, Goumon Y, Lugardon K, Strub JM, Aunis D (1998) Antibacterial peptides are present in chromaffin cell secretory granules. Cell Mol Neurobiol 18:249–266
Crivellato E, Nico B, Ribatti D (2008) The chromaffin vesicle: advances in understanding the composition of a versatile, multifunctional secretory organelle. Anat Rec 291:1587–1602
Jiang JX, Gu S (2005) Gap junction- and hemichannel-independent actions of connexins. Biochim Biophys Acta 1711:208–214
Dbouk HA, Mroue RM, El-Sabban ME, Talhouk RS (2009) Connexins: a myriad of functions extending beyond assembly of gap junction channels. Cell Commun Signal 7:4
Li J, Habbes HW, Eiberger J, Willecke K, Dermietzel R, Meier C (2007) Analysis of connexin expression during mouse Schwann cell development identifies connexin29 as a novel marker for the transition of neural crest to precursor cells. Glia 55:93–103
Vogalis F, Hegg CC, Lucero MT (2005) Electrical coupling in sustentacular cells of the mouse olfactory epithelium. J Neurophysiol 94:1001–1012
Soji T, Herbert DC (1989) Intercellular communication between rat anterior pituitary cells. Anat Rec 224:523–533
Fauquier T, Lacampagne A, Travo P, Bauer K, Mollard P (2002) Hidden face of the anterior pituitary. Trends Endocrinol Metab 13:304–309
Rodriguez H, Filippa V, Mohamed F, Dominguez S, Scardapane L (2007) Interaction between chromaffin and sustentacular cells in adrenal medulla of viscacha (Lagostomus maximus maximus). Anat Histol Embryol 36:182–185
Douglas WW (1968) Stimulus-secretion coupling: the concept and clues from chromaffin and other cells. Br J Pharmacol 34:451–474
Wakade AR (1981) Studies on secretion of catecholamines evoked by acetylcholine or transmural stimulation of the rat adrenal gland. J Physiol 313:463–480
Chang Q, Pereda A, Pinter MJ, Balice-Gordon RJ (2000) Nerve injury induces gap junctional coupling among axotomized adult motor neurons. J Neurosci 20:674–684
Mentis GZ, Díaz E, Moran LB, Navarrete R (2002) Increased incidence of gap junctional coupling between spinal motoneurones following transient blockade of NMDA receptors in neonatal rats. J Physiol 544:757–764
Bornstein SR, Ehrhart-Bornstein M, Usadel H, Böckmann M, Scherbaum WA (1991) Morphological evidence for a close interaction of chromaffin cells with cortical cells within the adrenal gland. Cell Tissue Res 265:1–9
Schinner S, Bornstein SR (2005) Cortical–chromaffin cell interactions in the adrenal gland. Endocr Pathol 16:91–98
Sicard F, Ehrhart-Bornstein M, Corbeil D, Sperber S, Krug AW, Ziegler CG, Rettori V, McCann SM, Bornstein SR (2007) Age-dependent regulation of chromaffin cell proliferation by growth factors, dehydroepiandrosterone (DHEA), and DHEA sulfate. Proc Natl Acad Sci U S A 104:2007–2012
Bornstein SR, Ehrhart-Bornstein M, Scherbaum WA (1997) Morphological and functional studies of the paracrine interaction between cortex and medulla in the adrenal gland. Microsc Res Tech 36:520–533
Bornstein SR, Ehrhart-Bornstein M (1992) Ultrastructural evidence for a paracrine regulation of the rat adrenal cortex mediated by the local release of catecholamines from chromaffin cells. Endocrinology 131:3126–3128
Pohorecky LA, Piezzi RS, Wurtman RJ (1970) Steroid induction of phenylethanolamine-N-methyl transferase in adrenomedullary explants: independence of adrenal innervation. Endocrinology 86:1466–1468
Kandler K (1997) Coordination of neuronal activity by gap junctions in the developing neocortex. Semin Cell Dev Biol 8:43–51
Bruzzone R, Dermietzel R (2006) Structure and function of gap junctions in the developing brain. Cell Tissue Res 326:239–248
Seidler FJ, Slotkin TA (1985) Adrenomedullary function in the neonatal rat: responses to acute hypoxia. J Physiol 358:1–16
Slotkin TA, Seidler FJ (1988) Adrenomedullary catecholamine release in the fetus and newborn: secretory mechanisms and their role in stress and survival. J Dev Physiol 10:1–16
García-Fernández M, Mejías R, López-Barneo J (2007) Developmental changes of chromaffin cell secretory response to hypoxia studied in thin adrenal slices. Pflügers Arch 454:93–100
Rico AJ, Prieto-Lloret J, Gonzalez C, Rigual R (2005) Hypoxia and acidosis increase the secretion of catecholamines in the neonatal rat adrenal medulla: an in vitro study. Am J Physiol Cell Physiol 289:C1417–C1425
Thompson RJ, Buttigieg J, Zhang M, Nurse CA (2007) A rotenone-sensitive site and H2O2 are key components of hypoxia-sensing in neonatal rat adrenomedullary chromaffin cells. Neuroscience 145:130–141
Spray DC, Bennett MV (1985) Physiology and pharmacology of gap junctions. Annu Rev Physiol 47:281–303
Alexander DB, Goldberg GS (2003) Transfer of biologically important molecules between cells through gap junction channels. Curr Med Chem 10:2045–2058
Upham BL, Trosko JE (2009) Oxidative-dependent integration of signal transduction with intercellular gap junctional communication in the control of gene expression. Antioxid Redox Signal 11:297–307
Henion PD, Landis SC (1990) Asynchronous appearance and topographic segregation of neuropeptide-containing cells in the developing rat adrenal medulla. J Neurosci 10:2886–2896
Huber K, Combs S, Ernsberger U, Kalcheim C, Unsicker K (2002) Generation of neuroendocrine chromaffin cells from sympathoadrenal progenitors: beyond the glucocorticoid hypothesis. Ann N Y Acad Sci 971:554–559
Colomer C, Lafont C, Guérineau NC (2008b) Stress-induced intercellular communication remodeling in the rat adrenal medulla. Ann N Y Acad Sci 1148:106–111
Collares-Buzato CB, Leite AR, Boschero AC (2001) Modulation of gap and adherent junctional proteins in cultured neonatal pancreatic islets. Pancreas 23:177–185
Leite AR, Carvalho CP, Furtado AG, Barbosa HC, Boschero AC, Collares-Buzato CB (2005) Co-expression and regulation of connexins 36 and 43 in cultured neonatal rat pancreatic islets. Can J Physiol Pharmacol 83:142–151
Maubert E, Tramu G, Croix D, Beauvillain J-C, Dupouy J-P (1990) Co-localization of vasoactive intestinal polypeptide and neuropeptide Y immunoreactivities in the nerve fibers of the rat adrenal gland. Neurosci Lett 113:121–126
Wakade TD, Blank MA, Malhotra RK, Pourcho R, Wakade AR (1991) The peptide VIP is a neurotransmitter in rat adrenal medulla: physiological role in controlling catecholamine secretion. J Physiol 444:349–362
Guo X, Wakade AR (1994) Differential secretion of catecholamines in response to peptidergic and cholinergic transmitters in rat adrenals. J Physiol 475:539–545
Zimmermann H (1994) Signalling via ATP in the nervous system. Trends Neurosci 17:420–426
Marley PD, McLeod J, Anderson C, Thomson KA (1995) Nerves containing nitric oxide synthase and their possible function in the control of catecholamine secretion in the bovine adrenal medulla. J Auton Nerv Syst 54:184–194
Malhotra RK, Wakade AR (1987) Non-cholinergic component of rat splanchnic nerves predominates at low neuronal activity and is eliminated by naloxone. J Physiol 383:639–652
Ngezahayo A, Kolb HA (1994) Regulation of gap junctional coupling in isolated pancreatic acinar cell pairs by cholecystokinin-octapeptide, vasoactive intestinal peptide (VIP) and a VIP-antagonist. J Membr Biol 139:127–136
Rörig B, Sutor B (1996) Regulation of gap junction coupling in the developing neocortex. Mol Neurobiol 12:225–249
Baldridge WH, Vaney DI, Weiler R (1998) The modulation of intercellular coupling in the retina. Semin Cell Dev Biol 9:311–318
Hotz-Wagenblatt A, Shalloway D (1993) Gap junctional communication and neoplastic transformation. Crit Rev Oncog 4:541–558
Czyz J (2008) The stage-specific function of gap junctions during tumourigenesis. Cell Mol Biol Lett 13:92–102
Yamasaki H, Mesnil M, Omori Y, Mironov N, Krutovskikh V (1995) Intercellular communication and carcinogenesis. Mutat Res 333:181–188
King TJ, Lampe PD (2004) Mice deficient for the gap junction protein Connexin32 exhibit increased radiation-induced tumorigenesis associated with elevated mitogen-activated protein kinase (p44/Erk1, p42/Erk2) activation. Carcinogenesis 25:669–680
Lampe PD, Lau AF (2004) The effects of connexin phosphorylation on gap junctional communication. Int J Biochem Cell Biol 36:1171–1186
Trosko JE, Ruch RJ (2002) Gap junctions as targets for cancer chemoprevention and chemotherapy. Curr Drug Targets 3:465–482
Pointis G, Fiorini C, Gilleron J, Carette D, Segretain D (2007) Connexins as precocious markers and molecular targets for chemical and pharmacological agents in carcinogenesis. Curr Med Chem 14:2288–2303
Tischler AS (2008) Pheochromocytoma and extra-adrenal paraganglioma: updates. Arch Pathol Lab Med 132:1272–1284
King TJ, Gurley KE, Prunty J, Shin JL, Kemp CJ, Lampe PD (2005) Deficiency in the gap junction protein connexin32 alters p27Kip1 tumor suppression and MAPK activation in a tissue-specific manner. Oncogene 24:1718–1726
Murray SA, Davis K, Fishman LM, Bornstein SR (2000) Alpha1 connexin 43 gap junctions are decreased in human adrenocortical tumors. J Clin Endocrinol Metab 85:890–895
Umhauer S, Ruch RJ, Fanning J (2000) Gap junctional intercellular communication and connexin 43 expression in ovarian carcinoma. Am J Obstet Gynecol 182:999–1000
Naoi Y, Miyoshi Y, Taguchi T, Kim SJ, Arai T, Maruyama N, Tamaki Y, Noguchi S (2008) Connexin26 expression is associated with aggressive phenotype in human papillary and follicular thyroid cancers. Cancer Lett 262:248–256
Fulop T, Smith C (2007) Matching native electrical stimulation by graded chemical stimulation in isolated mouse adrenal chromaffin cells. J Neurosci Methods 166:195–202
Hatton GI (1998) Synaptic modulation of neuronal coupling. Cell Biol Int 22:765–780
Wakade AR (1998) Multiple transmitter control of catecholamine secretion in rat adrenal medulla. Adv Pharmacol 42:595–598
Dun NJ, Tang H, Dun SL, Huang R, Dun EC, Wakade AR (1996) Pituitary adenylate cyclase activating polypeptide-immunoreactive sensory neurons innervate rat adrenal medulla. Brain Res 716:11–21
Murray SA, Davis K, Gay V (2003) ACTH and adrenocortical gap junctions. Microsc Res Tech 61:240–246
De Vries G, Sherman A (2000) Channel sharing in pancreatic beta-cells revisited: enhancement of emergent bursting by noise. J Theor Biol 207:513–530
Douglas WW, Poisner AM, Rubin RP (1965) Efflux of adenine nucleotides from perfused adrenal glands exposed to nicotine and other chromaffin cell stimulants. J Physiol 179:130–137
Douglas WW, Poisner AM (1966) Evidence that the secreting adrenal chromaffin cell releases catecholamines directly from ATP-rich granules. J Physiol 183:236–248
Douglas WW, Kanno T, Sampson SR (1967) Effects of acetylcholine and other medullary secretagogues and antagonists on the membrane potential of adrenal chromaffin cells: an analysis employing techniques of tissue culture. J Physiol 188:107–120
Douglas WW, Kanno T, Sampson SR (1967) Influence of the ionic environment on the membrane potential of adrenal chromaffin cells and on the depolarizing effect of acetylcholine. J Physiol 191:107–121
Akiyama T, Yamazaki T, Mori H, Sunagawa K (2004) Simultaneous monitoring of acetylcholine and catecholamine release in the in vivo rat adrenal medulla. Neurochem Int 44:497–503
Acknowledgments
The authors thank Drs. Dominique Aunis and François Molino for critical reading of the manuscript and Mireille Passama for help with the artwork. This work has been supported by the Centre National de la Recherche Scientifique, Institut National de la Santé et de la Recherche Médicale, Ministère de l’Enseignement Supérieur et de la Recherche, Fondation pour la Recherche Médicale, ARC Régionale, and Région Languedoc-Roussillon.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Colomer, C., Desarménien, M.G. & Guérineau, N.C. Revisiting the Stimulus-Secretion Coupling in the Adrenal Medulla: Role of Gap Junction-Mediated Intercellular Communication. Mol Neurobiol 40, 87–100 (2009). https://doi.org/10.1007/s12035-009-8073-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-009-8073-0