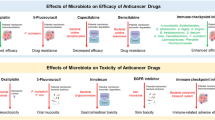
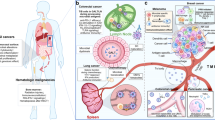
Abstract
In recent years, the role of gut microbiota in cancer treatment has attracted substantial attention. It is now well established that gut microbiota and its metabolites significantly contribute to the incidence, treatment, and prognosis of various cancers. This review provides a comprehensive review on the pivotal role of gut microbiota and their metabolites in cancer initiation and progression. Furthermore, it evaluates the impact of gut microbiota on the efficacy and associated side effects of anticancer therapies, including radiotherapy, chemotherapy, and immunotherapy, thus emphasizing the clinical importance of gut microbiota reconstitution in cancer treatment.
Similar content being viewed by others
Data availability
The data that support the findings of this study are available within the paper and its supplementary Information.
References
Brown EM, Sadarangani M, Finlay BB. The role of the immune system in governing host microbe interactions in the intestine. Nat Immunol. 2013;14(7):660–7. https://doi.org/10.1038/ni.2611.
Garrett WS. Cancer and the microbiota. Science. 2015;348(6230):80–6. https://doi.org/10.1126/science.aaa4972.
Sepich-Poore GD, Zitvogel L, Straussman R, Hasty J, Wargo JA, Knight R. The microbiome and human cancer. Science. 2021;371(6536):eabc4552. https://doi.org/10.1126/science.abc4552.
Zhang W, An Y, Qin X, Wu X, Wang X, Hou H, et al. Gut microbiota-derived metabolites in colorectal cancer: the bad and the challenges. Front Oncol. 2021;18(11):739648. https://doi.org/10.3389/fonc.2021.739648.
Sędzikowska A, Szablewski L. Human gut microbiota in health and selected cancers. Int J Mol Sci. 2021;22(24):13440. https://doi.org/10.3390/ijms222413440.
Zyoud SH, Al-Jabi SW, Amer R, Shakhshir M, Shahwan M, Jairoun AA, et al. Global research trends on the links between the gut microbiome and cancer: a visualization analysis. J Transl Med. 2022;20(1):83. https://doi.org/10.1186/s12967-022-03293-y.
Chen C-C, Liou J-M, Lee Y-C, Hong T-C, El-Omar EM, Wu M-S. The interplay between Helicobacter pylori and gastrointestinal microbiota. Gut Microbes. 2021;13(1):1–22. https://doi.org/10.1080/19490976.2021.1909459.
Jaye K, Li CG, Chang D, Bhuyan DJ. The role of key gut microbial metabolites in the development and treatment of cancer. Gut Microbes. 2022;14(1):2038865. https://doi.org/10.1080/19490976.2022.2038865.
Yang Q, Wang B, Zheng Q, Li H, Meng X, Zhou F, et al. A review of gut microbiota-derived meabolites in tumor progression and cancer therapy. Adv Sci Weinh Baden-Wurtt Ger. 2023;10(15):e2207366. https://doi.org/10.1002/advs.202207366.
Deleu S, Machiels K, Raes J, Verbeke K, Vermeire S. Short chain fatty acids and its producing organisms: an overlooked therapy for IBD? EBioMedicine. 2021;66:103293. https://doi.org/10.1016/j.ebiom.2021.103293.
Rangan P, Mondino A. Microbial short-chain fatty acids: a strategy to tune adoptive T cell therapy. J Immunother Cancer. 2022;10(7):e004147. https://doi.org/10.1136/jitc-2021-004147.
Gomes S, Rodrigues AC, Pazienza V, Preto A. Modulation of the tumor microenvironment by microbiota-derived short-chain fatty acids: impact in colorectal cancer therapy. Int J Mol Sci. 2023;24(6):5069. https://doi.org/10.3390/ijms24065069.
Hou H, Chen D, Zhang K, Zhang W, Liu T, Wang S, et al. Gut microbiota-derived short-chain fatty acids and colorectal cancer: ready for clinical translation? Cancer Lett. 2022;526:225–35. https://doi.org/10.1016/j.canlet.2021.11.027.
Geng H-W, Yin F-Y, Zhang Z-F, Gong X, Yang Y. Butyrate suppresses glucose metabolism of colorectal cancer cells via gpr109a-akt signaling pathway and enhances chemotherapy. Front Mol Biosci. 2021;8:634874. https://doi.org/10.3389/fmolb.2021.634874.
Huo R-X, Wang Y-J, Hou S-B, Wang W, Zhang C-Z, Wan X-H. Gut mucosal microbiota profiles linked to colorectal cancer recurrence. World J Gastroenterol. 2022;28(18):1946–64. https://doi.org/10.3748/wjg.v28.i18.1946.
Coutzac C, Jouniaux J-M, Paci A, Schmidt J, Mallardo D, Seck A, et al. Systemic short chain fatty acids limit antitumor effect of CTLA-4 blockade in hosts with cancer. Nat Commun. 2020;11(1):2168. https://doi.org/10.1038/s41467-020-16079-x.
Panebianco C, Villani A, Pisati F, Orsenigo F, Ulaszewska M, Latiano TP, et al. Butyrate, a postbiotic of intestinal bacteria, affects pancreatic cancer and gemcitabine response in in vitro and in vivo models. Biomed Pharmacother Biomedecine Pharmacother. 2022;151:113163. https://doi.org/10.1016/j.biopha.2022.113163.
Sanaei M, Kavoosi F. Effect of sodium butyrate on p16INK4a, p14ARF, p15INK4b, class I HDACs (HDACs 1, 2, 3) class II HDACs (HDACs 4, 5, 6), cell growth inhibition and apoptosis induction in pancreatic cancer AsPC-1 and colon cancer HCT-116 cell lines. Asian Pac J Cancer Prev. 2022;23(3):795–802. https://doi.org/10.31557/APJCP.2022.23.3.795.
Louis P, Hold GL, Flint HJ. The gut microbiota, bacterial metabolites and colorectal cancer. Nat Rev Microbiol. 2014;12(10):661–72. https://doi.org/10.1038/nrmicro3344.
Donohoe DR, Collins LB, Wali A, Bigler R, Sun W, Bultman SJ. The Warburg effect dictates the mechanism of butyrate-mediated histone acetylation and cell proliferation. Mol Cell. 2012;48(4):612–26. https://doi.org/10.1016/j.molcel.2012.08.033.
Okumura S, Konishi Y, Narukawa M, Sugiura Y, Yoshimoto S, Arai Y, et al. Gut bacteria identified in colorectal cancer patients promote tumourigenesis via butyrate secretion. Nat Commun. 2021;12(1):5674. https://doi.org/10.1038/s41467-021-25965-x.
Belcheva A, Irrazabal T, Robertson SJ, Streutker C, Maughan H, Rubino S, et al. Gut microbial metabolism drives transformation of MSH2-deficient colon epithelial cells. Cell. 2014;158(2):288–99. https://doi.org/10.1016/j.cell.2014.04.051.
Dicks LMT, Dreyer L, Smith C, van Staden AD. A review: the fate of bacteriocins in the human gastro-intestinal tract: do they cross the gut-blood barrier? Front Microbiol. 2018;9:2297. https://doi.org/10.3389/fmicb.2018.02297.
Dobson A, Cotter PD, Ross RP, Hill C. Bacteriocin production: a probiotic trait? Appl Environ Microbiol. 2012;78(1):1–6. https://doi.org/10.1128/AEM.05576-11.
Riboulet-Bisson E, Sturme MHJ, Jeffery IB, O’Donnell MM, Neville BA, Forde BM, et al. Effect of Lactobacillus salivarius bacteriocin Abp118 on the mouse and pig intestinal microbiota. PLoS ONE. 2012;7(2):e31113. https://doi.org/10.1371/journal.pone.0031113.
Kim T-S, Hur J-W, Yu M-A, Cheigh C-I, Kim K-N, Hwang J-K, et al. Antagonism of Helicobacter pylori by bacteriocins of lactic acid bacteria. J Food Prot. 2003;66(1):3–12. https://doi.org/10.4315/0362-028x-66.1.3.
Maher S, McClean S. Investigation of the cytotoxicity of eukaryotic and prokaryotic antimicrobial peptides in intestinal epithelial cells in vitro. Biochem Pharmacol. 2006;71(9):1289–98. https://doi.org/10.1016/j.bcp.2006.01.012.
Balcik-Ercin P, Sever B. An investigation of bacteriocin nisin anti-cancer effects and FZD7 protein interactions in liver cancer cells. Chem Biol Interact. 2022;366:110152. https://doi.org/10.1016/j.cbi.2022.110152.
Kaur J, Raza K, Preet S. Organogel mediated co-delivery of nisin and 5-fluorouracil: a synergistic approach against skin cancer. J Microencapsul. 2022;39(7–8):609–25. https://doi.org/10.1080/02652048.2022.2149871.
Patil SM, Barji DS, Aziz S, McChesney DA, Bagde S, Muttil P, et al. Pulmonary delivery of spray-dried Nisin ZP antimicrobial peptide for non-small cell lung cancer (NSCLC) treatment. Int J Pharm. 2023;634:122641. https://doi.org/10.1016/j.ijpharm.2023.122641.
Joo NE, Ritchie K, Kamarajan P, Miao D, Kapila YL. Nisin, an apoptogenic bacteriocin and food preservative, attenuates HNSCC tumorigenesis via CHAC1. Cancer Med. 2012;1(3):295–305. https://doi.org/10.1002/cam4.35.
Preet S, Bharati S, Panjeta A, Tewari R, Rishi P. Effect of nisin and doxorubicin on DMBA-inducedskin carcinogenesis—a possible adjunct therapy. Tumour Biol J Int Soc Oncodevelopmental Biol Med. 2015;36(11):8301–8. https://doi.org/10.1007/s13277-015-3571-3.
Avand A, Akbari V, Shafizadegan S. In vitro cytotoxic activity of a lactococcus lactis antimicrobial peptide against breast cancer cells. Iran J Biotechnol. 2018;16(3):e1867. https://doi.org/10.15171/ijb.1867.
Duboc H, Rajca S, Rainteau D, Benarous D, Maubert M-A, Quervain E, et al. Connecting dysbiosis, bile-acid dysmetabolism and gut inflammation in inflammatory bowel diseases. Gut. 2013;62(4):531–9. https://doi.org/10.1136/gutjnl-2012-302578.
Kriaa A, Mariaule V, Jablaoui A, Rhimi S, Mkaouar H, Hernandez J, et al. Bile acids: key players in inflammatory bowel diseases? Cells. 2022;11(5):901. https://doi.org/10.3390/cells11050901.
Bernstein C, Holubec H, Bhattacharyya AK, Nguyen H, Payne CM, Zaitlin B, et al. Carcinogenicity of deoxycholate, a secondary bile acid. Arch Toxicol. 2011;85(8):863–71. https://doi.org/10.1007/s00204-011-0648-7.
Liu L, Dong W, Wang S, Zhang Y, Liu T, Xie R, et al. Deoxycholic acid disrupts the intestinal mucosal barrier and promotes intestinal tumorigenesis. Food Funct. 2018;9(11):5588–97. https://doi.org/10.1039/c8fo01143e.
Payne CM, Weber C, Crowley-Skillicorn C, Dvorak K, Bernstein H, Bernstein C, et al. Deoxycholate induces mitochondrial oxidative stress and activates NF-kappaB through multiple mechanisms in HCT-116 colon epithelial cells. Carcinogenesis. 2007;28(1):215–22. https://doi.org/10.1093/carcin/bgl139.
Jin D, Huang K, Xu M, Hua H, Ye F, Yan J, et al. Deoxycholic acid induces gastric intestinal metaplasia by activating STAT3 signaling and disturbing gastric bile acids metabolism and microbiota. Gut Microbes. 2022;14(1):2120744. https://doi.org/10.1080/19490976.2022.2120744.
Ocvirk S, O’Keefe SJD. Dietary fat, bile acid metabolism and colorectal cancer. Semin Cancer Biol. 2021;73:347–55. https://doi.org/10.1016/j.semcancer.2020.10.003.
Hang S, Paik D, Yao L, Kim E, Trinath J, Lu J, et al. Author correction: bile acid metabolites control TH17 and Treg cell differentiation. Nature. 2020;579(7798):E7. https://doi.org/10.1038/s41586-020-2030-5.
Liu T, Song X, Khan S, Li Y, Guo Z, Li C, et al. The gut microbiota at the intersection of bile acids and intestinal carcinogenesis: an old story, yet mesmerizing. Int J Cancer. 2020;146:1780–90. https://doi.org/10.1002/ijc.32563.
Nguyen TT, Ung TT, Kim NH, Jung YD. Role of bile acids in colon carcinogenesis. World J Clin Cases. 2018;6(13):577–88. https://doi.org/10.12998/wjcc.v6.i13.577.
Mima K, Kosumi K, Baba Y, Hamada T, Baba H, Ogino S. The microbiome, genetics, and gastrointestinal neoplasms: the evolving field of molecular pathological epidemiology to analyze the tumor-immune-microbiome interaction. Hum Genet. 2021;140(5):725–46. https://doi.org/10.1007/s00439-020-02235-2.
Yoshimoto S, Loo TM, Atarashi K, Kanda H, Sato S, Oyadomari S, et al. Obesity-induced gut microbial metabolite promotes liver cancer through senescence secretome. Nature. 2013;499(7456):97–101. https://doi.org/10.1038/nature12347.
Goldberg AA, Titorenko VI, Beach A, Sanderson JT. Bile acids induce apoptosis selectively in androgen-dependent and -independent prostate cancer cells. PeerJ. 2013;1:e122. https://doi.org/10.7717/peerj.122.
Mikó E, Vida A, Kovács T, Ujlaki G, Trencsényi G, Márton J, et al. Lithocholic acid, a bacterial metabolite reduces breast cancer cell proliferation and aggressiveness. Biochim Biophys Acta Bioenerg. 2018;1859(9):958–74. https://doi.org/10.1016/j.bbabio.2018.04.002.
Surawicz CM, Brandt LJ, Binion DG, Ananthakrishnan AN, Curry SR, Gilligan PH, et al. Guidelines for diagnosis, treatment, and prevention of clostridium difficile infections. Am J Gastroenterol. 2013;108(4):478–98. https://doi.org/10.1038/ajg.2013.4.
Wang Y, Zhang S, Borody TJ, Zhang F. Encyclopedia of fecal microbiota transplantation: a review of effectiveness in the treatment of 85 diseases. Chin Med J (Engl). 2022;135(16):1927–39. https://doi.org/10.1097/CM9.0000000000002339.
Lu G, Wang W, Li P, Wen Q, Cui B, Zhang F. Washed preparation of faecal microbiota changes the transplantation related safety, quantitative method and delivery. Microb Biotechnol. 2022;15(9):2439–49. https://doi.org/10.1111/1751-7915.14074.
Marcella C, Cui B, Kelly CR, Ianiro G, Cammarota G, Zhang F. Systematic review: the global incidence of faecal microbiota transplantation-related adverse events from 2000 to 2020. Aliment Pharmacol Ther. 2021;53(1):33–42. https://doi.org/10.1111/apt.16148.
Rosshart SP, Vassallo BG, Angeletti D, Hutchinson DS, Morgan AP, Takeda K, et al. Wild mouse gut microbiota promotes host fitness and improves disease resistance. Cell. 2017;171(5):1015-1028.e13. https://doi.org/10.1016/j.cell.2017.09.016.
Wong SH, Zhao L, Zhang X, Nakatsu G, Han J, Xu W, et al. Gavage of fecal samples from patients with colorectal cancer promotes intestinal carcinogenesis in germ-free and conventional mice. Gastroenterology. 2017;153(6):1621-1633.e6. https://doi.org/10.1053/j.gastro.2017.08.022.
Routy B, Le Chatelier E, Derosa L, Duong CPM, Alou MT, Daillère R, et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science. 2018;359(6371):91–7. https://doi.org/10.1126/science.aan3706.
Badgeley A, Anwar H, Modi K, Murphy P, Lakshmikuttyamma A. Effect of probiotics and gut microbiota on anti-cancer drugs: Mechanistic perspectives. Biochim Biophys Acta Rev Cancer. 2021;1875(1):188494. https://doi.org/10.1016/j.bbcan.2020.188494.
Davar D, Dzutsev AK, McCulloch JA, Rodrigues RR, Chauvin J-M, Morrison RM, et al. Fecal microbiota transplant overcomes resistance to anti-PD-1 therapy in melanoma patients. Science. 2021;371(6529):595–602. https://doi.org/10.1126/science.abf3363.
Baruch EN, Youngster I, Ben-Betzalel G, Ortenberg R, Lahat A, Katz L, et al. Fecal microbiota transplant promotes response in immunotherapy-refractory melanoma patients. Science. 2021;371(6529):602–9. https://doi.org/10.1126/science.abb5920.
Ali H, Khurana S, Ma W, Peng Y, Jiang Z-D, DuPont H, et al. Safety and efficacy of fecal microbiota transplantation to treat and prevent recurrent Clostridioides difficile in cancer patients. J Cancer. 2021;12(21):6498–506. https://doi.org/10.7150/jca.59251.
Hefazi M, Patnaik MM, Hogan WJ, Litzow MR, Pardi DS, Khanna S. Safety and efficacy of fecal microbiota transplant for recurrent clostridium difficile infection in patients with cancer treated with cytotoxic chemotherapy: a single-institution retrospective case series. Mayo Clin Proc. 2017;92(11):1617–24. https://doi.org/10.1016/j.mayocp.2017.08.016.
Feuerstadt P, Louie TJ, Lashner B, Wang EEL, Diao L, Bryant JA, et al. SER-109, an oral microbiome therapy for recurrent clostridioides difficile infection. N Engl J Med. 2022;386(3):220–9. https://doi.org/10.1056/NEJMoa2106516.
Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, et al. Expert consensus document: the international scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. 2014;11(8):506–14. https://doi.org/10.1038/nrgastro.2014.66.
Goderska K, Agudo Pena S, Alarcon T. Helicobacter pylori treatment: antibiotics or probiotics. Appl Microbiol Biotechnol. 2018;102(1):1–7. https://doi.org/10.1007/s00253-017-8535-7.
Dahiya D, Nigam PS. Biotherapy using probiotics as therapeutic agents to restore the gut microbiota to relieve gastrointestinal tract inflammation, IBD, IBS and prevent induction of cancer. Int J Mol Sci. 2023;24(6):5748. https://doi.org/10.3390/ijms24065748.
Ghanavati R, Akbari A, Mohammadi F, Asadollahi P, Javadi A, Talebi M, et al. Lactobacillus species inhibitory effect on colorectal cancer progression through modulating the Wnt/β-catenin signaling pathway. Mol Cell Biochem. 2020;470(1–2):1–13. https://doi.org/10.1007/s11010-020-03740-8.
Wang T, Zheng J, Dong S, Ismael M, Shan Y, Wang X, et al. Lacticaseibacillus rhamnosus LS8 ameliorates azoxymethane/dextran sulfate sodium-induced colitis-associated tumorigenesis in mice via regulating gut microbiota and inhibiting inflammation. Probiotics Antimicrob Proteins. 2022;14(5):947–59. https://doi.org/10.1007/s12602-022-09967-9.
Fahmy CA, Gamal-Eldeen AM, El-Hussieny EA, Raafat BM, Mehanna NS, Talaat RM, et al. Bifidobacterium longum suppresses murine colorectal cancer through the modulation of oncomiRs and tumor suppressor miRNAs. Nutr Cancer. 2019;71(4):688–700. https://doi.org/10.1080/01635581.2019.1577984.
Wan L, Wu C, Wu Q, Luo S, Liu J, Xie X. Impact of probiotics use on clinical outcomes of immunecheckpoint inhibitors therapy in cancer patients. Cancer Med. 2023;12(2):1841–9. https://doi.org/10.1002/cam4.4994.
Tomita Y, Goto Y, Sakata S, Imamura K, Minemura A, Oka K, et al. Clostridium butyricum therapy restores the decreased efficacy of immune checkpoint blockade in lung cancer patients receiving proton pump inhibitors. Oncoimmunology. 2022;11(1):2081010. https://doi.org/10.1080/2162402X.2022.2081010.
Ye Z, Liang L, Xu Y, Yang J, Li Y. Probiotics influence gut microbiota and tumor immune microenvironment to enhance anti-tumor efficacy of doxorubicin. Probiotics Antimicrob Proteins. 2023. https://doi.org/10.1007/s12602-023-10073-7.
Kim S, Kim Y, Lee S, Kim Y, Jeon B, Kim H, et al. Live biotherapeutic Lactococcus lactis GEN3013 enhances antitumor efficacy of cancer treatment via modulation of cancer progression and immune system. Cancers. 2022;14(17):4083. https://doi.org/10.3390/cancers14174083.
Wang F, Yin Q, Chen L, Davis MM. Bifidobacterium can mitigate intestinal immunopathology in the context of CTLA-4 blockade. Proc Natl Acad Sci USA. 2018;115(1):157–61. https://doi.org/10.1073/pnas.1712901115.
Keen EC, Dantas G. Close encounters of three kinds: bacteriophages, commensal bacteria, and host immunity. Trends Microbiol. 2018;26(11):943–54. https://doi.org/10.1016/j.tim.2018.05.009.
Koskella B, Brockhurst MA. Bacteria-phage coevolution as a driver of ecological and evolutionary processes in microbial communities. FEMS Microbiol Rev. 2014;38(5):916–31. https://doi.org/10.1111/1574-6976.12072.
Hsu BB, Gibson TE, Yeliseyev V, Liu Q, Lyon L, Bry L, et al. Dynamic modulation of the gut microbiota and metabolome by bacteriophages in a mouse model. Cell Host Microbe. 2019;25(6):803-814.e5. https://doi.org/10.1016/j.chom.2019.05.001.
Zheng D-W, Dong X, Pan P, Chen K-W, Fan J-X, Cheng S-X, et al. Phage-guided modulation of the gut microbiota of mouse models of colorectal cancer augments their responses to chemotherapy. Nat Biomed Eng. 2019;3(9):717–28. https://doi.org/10.1038/s41551-019-0423-2.
Oster P, Vaillant L, Riva E, McMillan B, Begka C, Truntzer C, et al. Helicobacter pylori infection has a detrimental impact on the efficacy of cancer immunotherapies. Gut. 2022;71(3):457–66. https://doi.org/10.1136/gutjnl-2020-323392.
Islam MR, Arthur S, Haynes J, Butts MR, Nepal N, Sundaram U. The role of gut microbiota and metabolites in obesity-associated chronic gastrointestinal disorders. Nutrients. 2022;14(3):624. https://doi.org/10.3390/nu14030624.
Gentile CL, Weir TL. The gut microbiota at the intersection of diet and human health. Science. 2018;362(6416):776–80. https://doi.org/10.1126/science.aau5812.
Bradbury KE, Murphy N, Key TJ. Diet and colorectal cancer in UK Biobank: a prospective study. Int J Epidemiol. 2020;49(1):246–58. https://doi.org/10.1093/ije/dyz064.
Spencer CN, McQuade JL, Gopalakrishnan V, McCulloch JA, Vetizou M, Cogdill AP, et al. Dietary fiber and probiotics influence the gut microbiome and melanoma immunotherapy response. Science. 2021;374(6575):1632–40. https://doi.org/10.1126/science.aaz7015.
Song M, Chan AT. Environmental factors, gut microbiota, and colorectal cancer prevention. Clin Gastroenterol Hepatol Off Clin Pract J Am Gastroenterol Assoc. 2019;17(2):275–89. https://doi.org/10.1016/j.cgh.2018.07.012.
Wirth M, Joachim J, Tooze SA. Autophagosome formation–the role of ULK1 and Beclin1-PI3KC3 complexes in setting the stage. Semin Cancer Biol. 2013;23(5):301–9. https://doi.org/10.1016/j.semcancer.2013.05.007.
Huang B, Gui M, Ni Z, He Y, Zhao J, Peng J, et al. Chemotherapeutic drugs induce different gut microbiota disorder pattern and NOD/RIP2/NF-κb signaling pathway activation that lead to different degrees of intestinal injury. Microbiol Spectr. 2022;10(6):e0167722. https://doi.org/10.1128/spectrum.01677-22.
Daillère R, Vétizou M, Waldschmitt N, Yamazaki T, Isnard C, Poirier-Colame V, et al. Enterococcus hirae and barnesiella intestinihominis facilitate cyclophosphamide-induced therapeutic immunomodulatory effects. Immunity. 2016;45(4):931–43. https://doi.org/10.1016/j.immuni.2016.09.009.
Picard M, Yonekura S, Slowicka K, Petta I, Rauber C, Routy B, et al. Ileal immune tonus is a prognosis marker of proximal colon cancer in mice and patients. Cell Death Differ. 2021;28(5):1532–47. https://doi.org/10.1038/s41418-020-00684-w.
Roberti MP, Yonekura S, Duong CPM, Picard M, Ferrere G, Tidjani Alou M, et al. Chemotherapy-induced ileal crypt apoptosis and the ileal microbiome shape immunosurveillance and prognosis of proximal colon cancer. Nat Med. 2020;26(6):919–31. https://doi.org/10.1038/s41591-020-0882-8.
Yu T, Guo F, Yu Y, Sun T, Ma D, Han J, et al. Fusobacterium nucleatum promotes chemoresistance to colorectal cancer by modulating autophagy. Cell. 2017;170(3):548-563.e16. https://doi.org/10.1016/j.cell.2017.07.008.
Xie Y-H, Gao Q-Y, Cai G-X, Sun X-M, Zou T-H, Chen H-M, et al. Fecal clostridium symbiosum for noninvasive detection of early and advanced colorectal cancer: test and validation studies. EBioMedicine. 2017;25:32–40. https://doi.org/10.1016/j.ebiom.2017.10.005.
Yuan L, Zhang S, Li H, Yang F, Mushtaq N, Ullah S, et al. The influence of gut microbiota dysbiosis to the efficacy of 5-Fluorouracil treatment on colorectal cancer. Biomed Pharmacother Biomedecine Pharmacother. 2018;108:184–93. https://doi.org/10.1016/j.biopha.2018.08.165.
Tesniere A, Schlemmer F, Boige V, Kepp O, Martins I, Ghiringhelli F, et al. Immunogenic death of colon cancer cells treated with oxaliplatin. Oncogene. 2010;29(4):482–91. https://doi.org/10.1038/onc.2009.356.
Imai H, Saijo K, Komine K, Yoshida Y, Sasaki K, Suzuki A, et al. Antibiotics improve the treatment efficacy of oxaliplatin-based but not irinotecan-based therapy in advanced colorectal cancer patients. J Oncol. 2020;2020:1701326. https://doi.org/10.1155/2020/1701326.
Feather CE, Lees JG, Makker PGS, Goldstein D, Kwok JB, Moalem-Taylor G, et al. Oxaliplatin induces muscle loss and muscle-specific molecular changes in Mice. Muscle Nerve. 2018;57(4):650–8. https://doi.org/10.1002/mus.25966.
Wang J, Feng W, Zhang S, Chen L, Tang F, Sheng Y, et al. Gut microbial modulation in the treatment of chemotherapy-induced diarrhea with Shenzhu capsule. BMC Complement Altern Med. 2019;19(1):126. https://doi.org/10.1186/s12906-019-2548-y.
Wang Y, Sun L, Chen S, Guo S, Yue T, Hou Q, et al. The administration of Escherichia coli Nissle 1917 ameliorates irinotecan–induced intestinal barrier dysfunction and gut microbial dysbiosis in mice. Life Sci. 2019;231:116529. https://doi.org/10.1016/j.lfs.2019.06.004.
Yuan W, Xiao X, Yu X, Xie F, Feng P, Malik K, et al. Probiotic therapy (BIO-THREE) mitigates intestinal microbial imbalance and intestinal damage caused by oxaliplatin. Probiotics Antimicrob Proteins. 2022;14(1):60–71. https://doi.org/10.1007/s12602-021-09795-3.
Chang C-W, Lee H-C, Li L-H, Chiang Chiau J-S, Wang T-E, Chuang W-H, et al. Fecal microbiota transplantation prevents intestinal injury, upregulation of toll-like receptors, and 5-fluorouracil/oxaliplatin-induced toxicity in colorectal cancer. Int J Mol Sci. 2020;21(2):386. https://doi.org/10.3390/ijms21020386.
Zhu H, Lu C, Gao F, Qian Z, Yin Y, Kan S, et al. Selenium-enriched Bifidobacterium longum DD98 attenuates irinotecan-induced intestinal and hepatic toxicity in vitro and in vivo. Biomed Pharmacother. 2021;143:112192. https://doi.org/10.1016/j.biopha.2021.112192.
Mahdy MS, Azmy AF, Dishisha T, Mohamed WR, Ahmed KA, Hassan A, et al. Irinotecan-gut microbiota interactions and the capability of probiotics to mitigate Irinotecan-associated toxicity. BMC Microbiol. 2023;23(1):53. https://doi.org/10.1186/s12866-023-02791-3.
Mármol I, Quero J, Rodríguez-Yoldi MJ, Cerrada E. Gold as a possible alternative to platinum-based chemotherapy for colon cancer treatment. Cancers. 2019;11(6):780. https://doi.org/10.3390/cancers11060780.
Shen S, Lim G, You Z, Ding W, Huang P, Ran C, et al. Gut microbiota is critical for the induction of chemotherapy-induced pain. Nat Neurosci. 2017;20(9):1213–6. https://doi.org/10.1038/nn.4606.
van der Hee B, Wells JM. Microbial regulation of host physiology by short-chain fatty acids. Trends Microbiol. 2021;29(8):700–12. https://doi.org/10.1016/j.tim.2021.02.001.
Poonacha KNT, Villa TG, Notario V. The interplay among radiation therapy, antibiotics and the microbiota: impact on cancer treatment outcomes. Antibiot Basel Switz. 2022;11(3):331. https://doi.org/10.3390/antibiotics11030331.
Leibowitz BJ, Wei L, Zhang L, Ping X, Epperly M, Greenberger J, et al. Ionizing irradiation induces acute haematopoietic syndrome and gastrointestinal syndrome independently in mice. Nat Commun. 2014;5:3494. https://doi.org/10.1038/ncomms4494.
Zhao T-S, Xie L-W, Cai S, Xu J-Y, Zhou H, Tang L-F, et al. Dysbiosis of gut microbiota is associated with the progression of radiation-induced intestinal injury and is alleviated by oral compound probiotics in mouse model. Front Cell Infect Microbiol. 2021;11:717636. https://doi.org/10.3389/fcimb.2021.717636.
Reis Ferreira M, Andreyev HJN, Mohammed K, Truelove L, Gowan SM, Li J, et al. Microbiota and radiotherapy-induced gastrointestinal side-effects (MARS) study: a large pilot study of the microbiome in acute and late-radiation enteropathy. Clin Cancer Res. 2019;25(21):6487–500. https://doi.org/10.1158/1078-0432.CCR-19-0960.
Chen Z-Y, Xiao H-W, Dong J-L, Li Y, Wang B, Fan S-J, et al. Gut microbiota-derived PGF2α fights against radiation-induced lung toxicity through the MAPK/NF-κB pathway. Antioxidants. 2021;11(1):65. https://doi.org/10.3390/antiox11010065.
Ding X, Li Q, Li P, Chen X, Xiang L, Bi L, et al. Fecal microbiota transplantation: a promising treatment for radiation enteritis? Radiother Oncol. 2020;143:12–8. https://doi.org/10.1016/j.radonc.2020.01.011.
Cui M, Xiao H, Li Y, Zhou L, Zhao S, Luo D, et al. Faecal microbiota transplantation protects against radiation-induced toxicity. EMBO Mol Med. 2017;9(4):448–61. https://doi.org/10.15252/emmm.201606932.
Xiao H, Fan Y, Li Y, Dong J, Zhang S, Wang B, et al. Oral microbiota transplantation fights against head and neck radiotherapy-induced oral mucositis in mice. Comput Struct Biotechnol J. 2021;19:5898–910. https://doi.org/10.1016/j.csbj.2021.10.028.
Jian Y-P, Yang G, Zhang L-H, Liang J-Y, Zhou H-L, Wang Y-S, et al. Lactobacillus plantarum alleviates irradiation-induced intestinal injury by activation of FXR-FGF15 signaling in intestinal epithelia. J Cell Physiol. 2022;237(3):1845–56. https://doi.org/10.1002/jcp.30651.
Riehl TE, Alvarado D, Ee X, Zuckerman A, Foster L, Kapoor V, et al. Lactobacillus rhamnosus GG protects the intestinal epithelium from radiation injury through release of lipoteichoic acid, macrophage activation and the migration of mesenchymal stem cells. Gut. 2019;68(6):1003–13. https://doi.org/10.1136/gutjnl-2018-316226.
Ting NL-N, Lau HC-H, Yu J. Cancer pharmacomicrobiomics: targeting microbiota to optimise cancer therapy outcomes. Gut. 2022;71(7):1412–25. https://doi.org/10.1136/gutjnl-2021-326264.
Derosa L, Routy B, Thomas AM, Iebba V, Zalcman G, Friard S, et al. Intestinal akkermansia muciniphila predicts clinical response to Pd-1 blockade in patients with advanced non-small-cell lung cancer. Nat Med. 2022;28(2):315–24. https://doi.org/10.1038/s41591-021-01655-5.
Sivan A, Corrales L, Hubert N, Williams JB, Aquino-Michaels K, Earley ZM, et al. Commensal bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science. 2015;350(6264):1084–9. https://doi.org/10.1126/science.aac4255.
Gao G, Shen S, Zhang T, Zhang J, Huang S, Sun Z, et al. Lacticaseibacillus rhamnosus probio-M9enhanced the antitumor response to anti-PD-1 therapy by modulating intestinal metabolites. EBioMedicine. 2023;91:104533. https://doi.org/10.1016/j.ebiom.2023.104533.
Vétizou M, Pitt JM, Daillère R, Lepage P, Waldschmitt N, Flament C, et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science. 2015;350(6264):1079–84. https://doi.org/10.1126/science.aad1329.
Che H, Xiong Q, Ma J, Chen S, Wu H, Xu H, et al. Association of Helicobacter pylori infection with survival outcomes in advanced gastric cancer patients treated with immune checkpoint inhibitors. BMC Cancer. 2022;22(1):904. https://doi.org/10.1186/s12885-022-10004-9.
Pinato DJ, Howlett S, Ottaviani D, Urus H, Patel A, Mineo T, et al. Association of prior antibiotic treatment with survival and response to immune checkpoint inhibitor therapy in patients with cancer. JAMA Oncol. 2019;5(12):1774–8. https://doi.org/10.1001/jamaoncol.2019.2785.
Cheung KS, Lam LK, Seto WK, Leung WK. Use of Antibiotics during immune checkpoint inhibitor treatment is associated with lower survival in hepatocellular carcinoma. Liver Cancer. 2021;10(6):606–14. https://doi.org/10.1159/000518090.
Giordan Q, Salleron J, Vallance C, Moriana C, Clement-Duchene C. Impact of antibiotics and proton pump inhibitors on efficacy and tolerance of anti-PD-1 immune checkpoint inhibitors. Front Immunol. 2021;12:716317. https://doi.org/10.3389/fimmu.2021.716317.
Ochi N, Ichihara E, Takigawa N, Harada D, Inoue K, Shibayama T, et al. The effects of antibioticson the efficacy of immune checkpoint inhibitors in patients with non–small-cell lung cancer differ based on PD-L1 expression. Eur J Cancer. 2021;149:73–81. https://doi.org/10.1016/j.ejca.2021.02.040.
Zitvogel L, Ma Y, Raoult D, Kroemer G, Gajewski TF. The microbiome in cancer immunotherapy: diagnostic tools and therapeutic strategies. Science. 2018;359(6382):1366–70. https://doi.org/10.1126/science.aar6918.
Wang Y, Wiesnoski DH, Helmink BA, Gopalakrishnan V, Choi K, DuPont HL, et al. Fecal microbiota transplantation for refractory immune checkpoint inhibitor-associated colitis. Nat Med. 2018;24(12):1804–8. https://doi.org/10.1038/s41591-018-0238-9.
Funding
The authors did not receive support from any organization for the submitted work.
Author information
Authors and Affiliations
Contributions
The first draft of the article was written by YC and all authors commented on previous versions of the article. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
Not required as this was a systematic review of published studies.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, Y., Wang, X., Ye, Y. et al. Gut microbiota in cancer: insights on microbial metabolites and therapeutic strategies. Med Oncol 41, 25 (2024). https://doi.org/10.1007/s12032-023-02249-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-023-02249-6