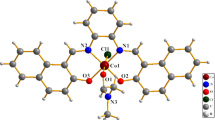
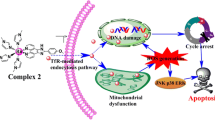
Abstract
Although chemotherapy has increased the life expectancy of cancer patients, its toxic side effects remain a major challenge. Recently, organometallic compounds, such as Schiff base copper complexes, have become promising candidates for next-generation anticancer drugs owing to their unique anticancer activities. In this study, binuclear copper(II) complex-1 and mononuclear copper(II) complex-2 were examined to analyze their anticancer mechanisms further. For this purpose, a viability test, flow cytometry analysis of apoptosis and the cell cycle, migration assay, and gene expression analysis were performed. According to our results, complex-1 was more cytotoxic than complex-2 at 24/48-h intervals. Our findings also demonstrated that both complexes induced apoptosis at IC50 concentrations and arrested the cell cycle at the G1-S checkpoint. However, complex-1 accelerates cell cycle arrest at the sub-G0/G1 phase more than complex-2 does. Furthermore, gene expression analysis showed that only complex-1 induces the expression of p53. Interestingly, both complexes induced Bcl-2 overexpression. However, they did not affect MMP-13 expression. More interestingly, both complexes inhibited cell migration in different ways, including amoeboid and collective, by recruiting protease-independent pathways. This study confirmed that adding several metal cores and co-ligands increased the activity of the complex. It also appeared that Cu-containing complexes could prevent the migration of cancer cells through protease-independent pathways, which can be used for novel therapeutic purposes.





Similar content being viewed by others
Data Availability
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials.
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49. https://doi.org/10.3322/caac.21660.
Debela DT, Muzazu SG, Heraro KD, Ndalama MT, Mesele BW, Haile DC, et al. New approaches and procedures for cancer treatment: Current perspectives. SAGE Open Med. 2021;9:20503121211034370. https://doi.org/10.1177/20503121211034366.
Di X, Bright AT, Bellott R, Gaskins E, Robert J, Holt S, et al. A chemotherapy-associated senescence bystander effect in breast cancer cells. Cancer Biol Ther. 2008;7(6):864–72. https://doi.org/10.4161/cbt.7.6.5861.
Malik MA, Dar OA, Gull P, Wani MY, Hashmi AA. Heterocyclic Schiff base transition metal complexes in antimicrobial and anticancer chemotherapy. Medchemcomm. 2018;9(3):409–36. https://doi.org/10.1039/c7md00526a.
Soroceanu A, Bargan A. Advanced and biomedical applications of Schiff-base ligands and their metal complexes: a review. Crystals. 2022;12(10):1436. https://doi.org/10.3390/cryst12101436.
Santini C, Pellei M, Gandin V, Porchia M, Tisato F, Marzano C. Advances in copper complexes as anticancer agents. Chem Rev. 2014;114(1):815–62. https://doi.org/10.1021/cr400135x.
Grubman A, White AR. Copper as a key regulator of cell signalling pathways. Expert Rev Mol Med. 2014;16:e11. https://doi.org/10.1017/erm.2014.11.
Mo Q, Deng J, Liu Y, Huang G, Li Z, Yu P, et al. Mixed-ligand Cu(II) hydrazone complexes designed to enhance anticancer activity. Eur J Med Chem. 2018;156:368–80. https://doi.org/10.1016/j.ejmech.2018.07.022.
Kergreis A, Lord RM, Pike SJ. Influence of ligand and nuclearity on the cytotoxicity of cyclometallated C^N^C platinum(II) complexes. Chemistry. 2020;26(65):14938–46. https://doi.org/10.1002/chem.202002517.
Fekri R, Salehi M, Asadi A, Kubicki M. DNA/BSA interaction, bio-activity, molecular docking simulation study and electrochemical properties of hydrazone Schiff base derived Cu(II)/Ni(II) metal complexes: Influence of the nuclearity and metal ions. Polyhedron. 2017;128:175–87. https://doi.org/10.1016/j.poly.2017.02.047.
Fekri R, Salehi M, Asadi A, Kubicki M. Synthesis, characterization, anticancer and antibacterial evaluation of Schiff base ligands derived from hydrazone and their transition metal complexes. Inorg Chim Acta. 2019;484:245–54. https://doi.org/10.1016/j.ica.2018.09.022.
Fekri R, Salehi M, Asadi A, Kubicki M. Spectroscopic studies, structural characterization and electrochemical studies of two cobalt (III) complexes with tridentate hydrazone Schiff base ligands: Evaluation of antibacterial activities, DNA-binding, BSA interaction and molecular docking. Appl Organomet Chem. 2018;32(2):e4019. https://doi.org/10.1002/aoc.4019.
Lintz M, Muñoz A, Reinhart-King CA. The mechanics of single cell and collective migration of tumor cells. J Biomech Eng. 2017;139(2):0210051–9. https://doi.org/10.1115/1.4035121.
Baranwal BP, Tripathi K, Singh AK, Tripathi S. Synthesis and spectral characterization of ternary mixed-vanadyl β-diketonate complexes with Schiff bases. Spectrochim Acta A Mol Biomol Spectrosc. 2012;91:365–9. https://doi.org/10.1016/j.saa.2012.02.017.
Habibi MH, Shojaee E, Nichol GS. Synthesis, spectroscopic characterization and crystal structure of novel NNNN-donor μ-bis(bidentate) tetraaza acyclic Schiff base ligands. Spectrochim Acta A Mol Biomol Spectrosc. 2012;98:396–404. https://doi.org/10.1016/j.saa.2012.08.064.
Madhu V, Sabbani S, Kishore R, Naik IK, Das SK. Mechanical motion in the solid state and molecular recognition: reversible cis–trans transformation of an organic receptor in a solid–liquid crystalline state reaction triggered by anion exchange. CrystEngComm. 2015;17(17):3219–23. https://doi.org/10.1039/C5CE00449G.
Adhikary J, Chakraborty P, Das B, Datta A, Dash SK, Roy S, et al. Preparation and characterization of ferromagnetic nickel oxide nanoparticles from three different precursors: application in drug delivery. RSC Adv. 2015;5(45):35917–28. https://doi.org/10.1039/C5RA00642B.
Arjmand F, Sayeed F, Muddassir M. Synthesis of new chiral heterocyclic Schiff base modulated Cu(II)/Zn(II) complexes: their comparative binding studies with CT-DNA, mononucleotides and cleavage activity. J Photochem Photobiol B. 2011;103(2):166–79. https://doi.org/10.1016/j.jphotobiol.2011.03.001.
Patel RN, Singh YP, Singh Y, Butcher RJ, Zeller M. Unprecedented copper(II) mediated in situ formation of gem-diol binuclear complexes: a combined experimental and computational study. RSC Adv. 2016;6(109):107379–98. https://doi.org/10.1039/C6RA20367A.
Shakdofa MM, Shtaiwi MH, Morsy N, Abdel-rassel T. Metal complexes of hydrazones and their biological, analytical and catalytic applications: A review. Main Group Chem. 2014;13(3):187–218. https://doi.org/10.1002/CHIN.201506320.
Graf N, Lippard SJ. Redox activation of metal-based prodrugs as a strategy for drug delivery. Adv Drug Deliv Rev. 2012;64(11):993–1004. https://doi.org/10.1016/j.addr.2012.01.007.
Gandin V, Tisato F, Dolmella A, Pellei M, Santini C, Giorgetti M, et al. In vitro and in vivo anticancer activity of copper(I) complexes with homoscorpionate tridentate tris(pyrazolyl)borate and auxiliary monodentate phosphine ligands. J Med Chem. 2014;57(11):4745–60. https://doi.org/10.1021/jm500279x.
Wang T, Guo Z. Copper in medicine: homeostasis, chelation therapy and antitumor drug design. Curr Med Chem. 2006;13(5):525–37. https://doi.org/10.2174/092986706776055742.
Qi J, Liang S, Gou Y, Zhang Z, Zhou Z, Yang F, et al. Synthesis of four binuclear copper(II) complexes: Structure, anticancer properties and anticancer mechanism. Eur J Med Chem. 2015;96:360–8. https://doi.org/10.1016/j.ejmech.2015.04.031.
Desbouis D, Troitsky IP, Belousoff MJ, Spiccia L, Graham B. Copper(II), zinc(II) and nickel(II) complexes as nuclease mimetics. Coord Chem Rev. 2012;256(11):897–937. https://doi.org/10.1016/j.ccr.2011.12.005.
Montagner D, Gandin V, Marzano C, Erxleben A. DNA damage and induction of apoptosis in pancreatic cancer cells by a new dinuclear bis(triazacyclonane) copper complex. J Inorg Biochem. 2015;145:101–7. https://doi.org/10.1016/j.jinorgbio.2015.01.013.
Majouga AG, Zvereva MI, Rubtsova MP, Skvortsov DA, Mironov AV, Azhibek DM, et al. Mixed valence copper(I, II) binuclear complexes with unexpected structure: synthesis, biological properties and anticancer activity. J Med Chem. 2014;57(14):6252–8. https://doi.org/10.1021/jm500154f.
Jany T, Moreth A, Gruschka C, Sischka A, Spiering A, Dieding M, et al. Rational design of a cytotoxic dinuclear Cu2 complex that binds by molecular recognition at two neighboring phosphates of the DNA backbone. Inorg Chem. 2015;54(6):2679–90. https://doi.org/10.1021/ic5028465.
Sinicropi MS, Ceramella J, Iacopetta D, Catalano A, Mariconda A, Rosano C, et al. Metal complexes with Schiff Bases: Data collection and recent studies on biological activities. Int J Mol Sci. 2022;23(23):14840. https://doi.org/10.3390/ijms232314840.
Marzano C, Pellei M, Tisato F, Santini C. Copper complexes as anticancer agents. Anticancer Agents Med Chem. 2009;9(2):185–211. https://doi.org/10.2174/187152009787313837.
Parasuraman B, Rajendran J, Rangappan R. An insight into antibacterial and anticancer activity of homo and hetero binuclear Schiff Base complexes. Orient J Chem. 2017;33(3):1223.
Jiang S, Ni H, Liu F, Gu S, Yu P, Gou Y. Binuclear Schiff base copper (II) complexes: Syntheses, crystal structures, HSA interaction and anti-cancer properties. Inorg Chim Acta. 2020;499: 119186.
Fekri R, Abdolmaleki A, Asadi A, Salehi M, Karimian A, Taghizadehmomen L, et al. Anticancer effects of copper (II) hydrazone Schiff base complex: A review. Basic Clin Cancer Res. 2022;13(2):143–55.
Akhmetova VR, Galimova E, Mescheryakova ES, Dzhemileva LU, Dzhemilev UM, D’Yakonov AV. Mono- and binuclear complexes of copper(II) with dimethylaminomethyl derivatives of 2-naphthol and 6-quinolinol: synthesis and in vitro study of antitumor properties. Metallomics. 2023;15(6):mfad037. https://doi.org/10.1093/mtomcs/mfad037.
Zhang Z, Wang H, Yan M, Wang H, Zhang C. Novel copper complexes as potential proteasome inhibitors for cancer treatment (Review). Mol Med Rep. 2017;15(1):3–11. https://doi.org/10.3892/mmr.2016.6022.
Molinaro C, Martoriati A, Pelinski L, Cailliau K. Copper complexes as anticancer agents targeting topoisomerases I and II. Cancers (Basel). 2020;12(10):2863. https://doi.org/10.3390/cancers12102863.
Hussain A, AlAjmi MF, Rehman MT, Amir S, Husain FM, Alsalme A, et al. Copper(II) complexes as potential anticancer and Nonsteroidal anti-inflammatory agents: In vitro and in vivo studies. Sci Rep. 2019;9(1):5237. https://doi.org/10.1038/s41598-019-41063-x.
Tchounwou PB, Newsome C, Williams J, Glass K. Copper-induced cytotoxicity and transcriptional activation of stress genes in human liver carcinoma (HepG(2)) cells. Met Ions Biol Med. 2008;10:285–90.
Lambert AW, Pattabiraman DR, Weinberg RA. Emerging biological principles of metastasis. Cell. 2017;168(4):670–91. https://doi.org/10.1016/j.cell.2016.11.037.
Bayat Mokhtari R, Homayouni TS, Baluch N, Morgatskaya E, Kumar S, Das B, et al. Combination therapy in combating cancer. Oncotarget. 2017;8(23):38022–43. https://doi.org/10.18632/oncotarget.16723.
Gandalovičová A, Rosel D, Fernandes M, Veselý P, Heneberg P, Čermák V, et al. Migrastatics-anti-metastatic and anti-invasion drugs: Promises and challenges. Trends Cancer. 2017;3(6):391–406. https://doi.org/10.1016/j.trecan.2017.04.008.
Balsa LM, Ruiz MC, Santa Maria de la Oarra L, Baran EJ, León IE. Anticancer and antimetastatic activity of copper(II)-tropolone complex against human breast cancer cells, breast multicellular spheroids and mammospheres. J Inorg Biochem. 2020;204:110975. https://doi.org/10.1016/j.jinorgbio.2019.110975.
Tsymbal S, Li G, Agadzhanian N, Sun Y, Zhang J, Dukhinova M, et al. Recent advances in copper-based organic complexes and nanoparticles for tumor theranostics. Molecules. 2022;27(20):7066. https://doi.org/10.3390/molecules27207066.
Cucci LM, Satriano C, Marzo T, La Mendola D. Angiogenin and copper crossing in wound healing. Int J Mol Sci. 2021;22(19):10704. https://doi.org/10.3390/ijms221910704.
Nasulewicz A, Mazur A, Opolski A. Role of copper in tumour angiogenesis–clinical implications. J Trace Elem Med Biol. 2004;18(1):1–8. https://doi.org/10.1016/j.jtemb.2004.02.004.
Ji P, Wang P, Chen H, Xu Y, Ge J, Tian Z, et al. Potential of copper and copper compounds for anticancer applications. Pharmaceuticals. 2023;16(2):234. https://doi.org/10.3390/ph16020234.
Jia Y, Chen L, Jia Q, Dou X, Xu N, Liao DJ. The well-accepted notion that gene amplification contributes to increased expression still remains, after all these years, a reasonable but unproven assumption. J Carcinog. 2016;15:3. https://doi.org/10.4103/1477-3163.182809.
Feroz W, Sheikh AMA. Exploring the multiple roles of guardian of the genome: P53. Egypt J Med Hum Genet. 2020;21(1):49. https://doi.org/10.1186/s43042-020-00089-x.
Chen J. The cell-cycle arrest and apoptotic functions of p53 in tumor initiation and progression. Cold Spring Harb Perspect Med. 2016;6(3):a026104. https://doi.org/10.1101/cshperspect.a026104.
Batchelor E, Loewer A, Mock C, Lahav G. Stimulus-dependent dynamics of p53 in single cells. Mol Syst Biol. 2011;7:488. https://doi.org/10.1038/msb.2011.20.
Geva-Zatorsky N, Rosenfeld N, Itzkovitz S, Milo R, Sigal A, Dekel E, et al. Oscillations and variability in the p53 system. Mol Syst Biol. 2006;2:2006.003. https://doi.org/10.1038/msb4100068.
Purvis JE, Karhohs KW, Mock C, Batchelor E, Loewer A, Lahav G. p53 dynamics control cell fate. Science. 2012;336(6087):1440–4. https://doi.org/10.1126/science.1218351.
El-Emshaty HM, Saad EA, Toson EA, Abdel Malak CA, Gadelhak NA. Apoptosis and cell proliferation: correlation with BCL-2 and P53 oncoprotein expression in human hepatocellular carcinoma. Hepatogastroenterology. 2014;61(133):1393–401. https://doi.org/10.5754/hge13453.
Tophkhane C, Yang S, Bales W, Archer L, Osunkoya A, Thor AD, et al. Bcl-2 overexpression sensitizes MCF-7 cells to genistein by multiple mechanisms. Int J Oncol. 2007;31(4):867–74.
Li S, Pritchard DM, Yu LG. Regulation and function of matrix metalloproteinase-13 in cancer progression and metastasis. Cancers (Basel). 2022;14(13):3263. https://doi.org/10.3390/cancers14133263.
González-Ballesteros MM, Mejía C, Ruiz-Azuara L. Metallodrugs: An approach against invasion and metastasis in cancer treatment. FEBS Open Bio. 2022;12(5):880–99. https://doi.org/10.1002/2211-5463.13381.
Sabeh F, Shimizu-Hirota R, Weiss SJ. Protease-dependent versus -independent cancer cell invasion programs: three-dimensional amoeboid movement revisited. J Cell Biol. 2009;185(1):11–9. https://doi.org/10.1083/jcb.200807195.
Friedl P, Wolf K. Plasticity of cell migration: A multiscale tuning model. J Cell Biol. 2010;188(1):11–9. https://doi.org/10.1083/jcb.200909003.
Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2(6):442–54. https://doi.org/10.1038/nrc822.
Ghasemi M, Turnbull T, Sebastian S, Kempson I. The MTT assay: Utility, limitations, pitfalls, and interpretation in bulk and single-cell analysis. Int J Mol Sci. 2021;22(23):12827. https://doi.org/10.3390/ijms222312827.
Crowley LC, Marfell BJ, Scott AP, Waterhouse NJ. Quantitation of apoptosis and necrosis by Annexin V binding, propidium iodide uptake, and flow cytometry. Cold Spring Harb Protoc. 2016. https://doi.org/10.1101/pdb.prot087288.
Pozarowski P, Darzynkiewicz Z. Analysis of cell cycle by flow cytometry. Methods Mol Biol. 2004;281:301–11. https://doi.org/10.1385/1-59259-811-0:301.
Liang CC, Park AY, Guan JL. In vitro scratch assay: a convenient and inexpensive method for analysis of cell migration in vitro. Nat Protoc. 2007;2(2):329–33. https://doi.org/10.1038/nprot.2007.30.
Funding
This work was supported by the Ardabil University of Medical Sciences. Funding organization had no role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study's conception and design. Y.P. performed material preparation, data collection, and analysis. The experiments were set up and carried out by V.A., M.A., Z.P., and R.F. The previous version of the manuscript was edited by A.A., K.N., and B.B. and commented on by all authors. Finally, Y.P. wrote and revised the entire manuscript for submission. All authors read and approved the final version of the manuscript for submission.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interest.
Ethical Approval
Ethical approval for this study was granted by the Ardabil University of Medical Sciences Ethics Committee Reference Number IR.ARUMS.REC.1399.295.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Asghariazar, V., Amini, M., Pirdel, Z. et al. The Schiff base hydrazine copper(II) complexes induce apoptosis by P53 overexpression and prevent cell migration through protease-independent pathways. Med Oncol 40, 271 (2023). https://doi.org/10.1007/s12032-023-02150-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-023-02150-2