Abstract
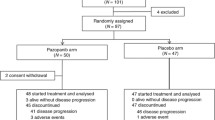
Pemetrexed is a key anticancer agent for treatment of advanced non-small cell lung cancer (NSCLC). Pemetrexed is generally well tolerated, but individual-patient differences exist in severity of adverse events. Our study aimed to characterize the adverse events of pemetrexed that result in discontinuation of chemotherapy and to identify risk factors associated with those adverse events. We retrospectively studied the incidence of adverse events in 257 patients with NSCLC who received pemetrexed (P) with or without bevacizumab (B) and/or carboplatin (C): P, PB, CP, or CPB. Patients whose chemotherapy was discontinued were divided into two groups according to adverse events and disease progression. Grade 2/3 nausea, fatigue with P and PB, and rash with CP and CPB occurred more frequent in the adverse events group than in the disease progression group. Multivariate analysis indicated that grade 2/3 nausea [odds ratio (OR) 9.94; 95% confidence interval (CI) 1.46–67.37; p = 0.01] and fatigue (OR 10.62; CI 1.60–70.20; p = 0.01) with P or PB, and rash (OR 6.12; CI 1.34–27.88; p = 0.01) with CP or CPB, were independent risk factors for discontinuation of chemotherapy. Administration of dexamethasone at doses less than 4 mg after the day of pemetrexed administration was associated with nausea following P or PB (OR 11.08; 95% CI 1.02–119.95; p = 0.04). Grade 2/3 nausea and fatigue with P or PB, and rash with CP or CPB, were associated with discontinuation of chemotherapy.
Similar content being viewed by others
References
Pujol JL, Paul S, Chouaki N, Peterson P, Moore P, Berry DA, et al. Survival without common toxicity criteria grade 3/4 toxicity for pemetrexed compared with docetaxel in previously treated patients with advanced non-small cell lung cancer (NSCLC): a risk-benefit analysis. J Thorac Oncol. 2007;2:397–401.
Khan S, Dhadda A, Fyfe D, Sundar S. Impact of neutropenia on delivering planned chemotherapy for solid tumours. Eur J Cancer Care. 2008;17:19–25.
Carmona-Bayonas A, Jiménez-Fonseca P, Virizuela Echaburu J, Antonio M, Font C, Biosca M, et al. Prediction of serious complications in patients with seemingly stable febrile neutropenia: validation of the Clinical Index of stable febrile neutropenia in a prospective cohort of patients from the FINITE study. J Clin Oncol. 2015;33:465–71.
Hurria A, Mohile S, Gajra A, Klepin H, Muss H, Chapman A, et al. Validation of a prediction tool for chemotherapy toxicity in older adults with cancer. J Clin Oncol. 2016;34:2366–71.
Scagliotti GV, Parikh P, von Pawel J, Biesma B, Vansteenkiste J, Manegold C, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol. 2008;26:3543–51.
Hanna N, Shepherd FA, Fossella FV, Pereira JR, De Marinis F, von Pawel J, et al. Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. J Clin Oncol. 2004;22:1589–97.
Basch E, Prestrud AA, Hesketh PJ, Kris MG, Feyer PC, Somerfield MR, et al. Antiemetics: American Society of clinical oncology clinical practice guideline update. J Clin Oncol. 2011;29:4189–98.
Kulkarni PM, Chen R, Anand T, Monberg MJ, Obasaju CK. Efficacy and safety of pemetrexed in elderly cancer patients: results of an integrated analysis. Crit Rev Oncol Hematol. 2008;67:64–70.
Zukin M, Barrios CH, Pereira JR, Ribeiro Rde A, Beato CA, do Nascimento YN, et al. Randomized phase III trial of single-agent pemetrexed versus carboplatin and pemetrexed in patients with advanced non-small-cell lung cancer and Eastern Cooperative Oncology Group performance status of 2. J Clin Oncol. 2013;31:2849–53.
Tomasini P, Barlesi F, Mascaux C, Greillier L. Pemetrexed for advanced stage nonsquamous non-small cell lung cancer latest evidence about its extended use and outcomes. Ther Adv Med Oncol. 2016;8:198–208.
McDonald A, Vasey P, Adams L, Walling J, Woodworth JR, Abrahams T, et al. A phase I and pharmacokinetic study of LY231514, the multitargeted antifolate. Clin Cancer Res. 1998;4:605–10.
Ando Y, Hayashi T, Ujita M, Murai S, Ohta H, Ito K, et al. Effect of renal function on pemetrexed-induced haematotoxicity. Cancer Chemother Pharmacol. 2016;78:183–9.
Ikesue H, Watanabe H, Hirano M, Chikamori A, Suetsugu K, Ryokai Y, et al. Risk factors for predicting severe neutropenia induced by pemetrexed plus carboplatin therapy in patients with advanced non-small cell lung cancer. Biol Pharm Bull. 2015;38:1192–8.
Kawazoe H, Yano A, Ishida Y, Takechi K, Katayama H, Ito R, et al. Non-steroidal anti-inflammatory drugs induce severe hematologic toxicities in lung cancer patients receiving pemetrexed plus carboplatin: a retrospective cohort study. PLoS ONE. 2017;12:e0171066. https://doi.org/10.1371/journal.pone.0171066.
Sakurada T, Kakiuchi S, Tajima S, Horinouchi Y, Konaka K, Okada N, et al. Pemetrexed-induced rash may be prevented by supplementary corticosteroids. Biol Pharm Bull. 2015;38:1752–6.
Colleoni M, Li S, Gelber RD, Price KN, Coates AS, Castiglione-Gertsch M, et al. Relation between chemotherapy dose, oestrogen receptor expression, and body-mass index. Lancet. 2005;366:1108–10.
Takeuchi H, Saeki T, Aiba K, Tamura K, Aogi K, Eguchi K, et al. Japanese society of clinical oncology clinical practice guidelines 2010 for antiemesis in oncology: executive summary. Int J Clin Oncol. 2016;21:1–12.
Adjei AA, Mandrekar SJ, Dy GK, Molina JR, Adjei AA, Gandara DR, et al. Phase II trial of pemetrexed plus bevacizumab for second-line therapy of patients with advanced non-small-cell lung cancer NCCTG and SWOG Study N0426. J Clin Oncol. 2010;28:614–9.
Paz-Ares L, de Marinis F, Dediu M, Thomas M, Pujol JL, Bidoli P, et al. Maintenance therapy with pemetrexed plus best supportive care versus placebo plus best supportive care after induction therapy with pemetrexed plus cisplatin for advanced non-squamous non-small-cell lung cancer (PARAMOUNT): a double-blind, phase 3, randomised controlled trial. Lancet Oncol. 2012;13:247–55.
Niyikiza C, Baker SD, Seitz DE, Walling JM, Nelson K, Rusthoven JJ, et al. Homocysteine and methylmalonic acid: markers to predict and avoid toxicity from pemetrexed therapy. Mol Cancer Ther. 2002;1:545–52.
Crawford J, Ozer H, Stoller R, Johnson D, Lyman G, Tabbara I, et al. Reduction by granulocyte colony-stimulating factor of fever and neutropenia induced by chemotherapy in patients with small-cell lung cancer. N Engl J Med. 1991;325:164–70.
Henzen C, Suter A, Lerch E, Urbinelli R, Schorno XH, Briner VA. Suppression and recovery of adrenal response after short-term, high-dose glucocorticoid treatment. Lancet. 2000;355:542–5.
Han HS, Shim YK, Kim JE, Jeon HJ, Lim SN, Oh TK, et al. A pilot study of adrenal suppression after dexamethasone therapy as an antiemetic in cancer patients. Support Care Cancer. 2012;20:1565–72.
Goricar K, Kovac V, Dolzan V. Polymorphisms in folate pathway and pemetrexed treatment outcome in patients with malignant pleural mesothelioma. Radiol Oncol. 2014;48:163–72.
Acknowledgements
We would like to thank the participating patients for their contribution to this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
S.K. and N.S.-A. have received research grants from SK, Eli Lilly, and Chugai Pharmaceutical for another research project.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. For this type of study, formal consent is not required.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Miyahara, T., Sueoka-Aragane, N., Iwanaga, K. et al. Severity and predictive factors of adverse events in pemetrexed-containing chemotherapy for non-small cell lung cancer. Med Oncol 34, 195 (2017). https://doi.org/10.1007/s12032-017-1053-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-017-1053-8