Abstract
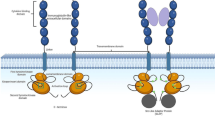
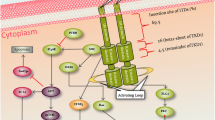
FMS-like tyrosine kinase 3 (FLT3) is a proto-oncogene involved in crucial steps of haematopoiesis such as proliferation, differentiation and survival. In recent years, FLT3 has been an important marker in different haematological malignancies, highlighting in acute myeloid leukaemia, where FLT3 mutations have been associated with the clinical prognosis, treatment and survival of patients. The most common form of FLT3 mutation is an internal tandem duplication (ITD) that promotes ligand-independent auto-phosphorylation and constitutive activation of the receptor. FLT3–ITD has been strongly associated with a bad prognosis, leukocytosis, high blast counts, increased risk of relapse and shorter overall survival. In order to improve the clinical condition of FLT3–ITD-positive patients, several FLT3 inhibitors have been developed showing variable results. Currently, the main challenges to be overcome are the different forms of resistance to FLT3 inhibitors. Thus, the purpose of this review is to present, in a general way, the current role that FLT3–ITD mutation plays in patients with AML, with a particular emphasis on the molecular mechanisms associated with clinical prognosis, treatment, and survival of patients.

Similar content being viewed by others
References
Rubnitz JE, Gibson B, Smith FO. Acute myeloid leukemia. Hematol Oncol Clin North Am Elsevier Ltd; 2010;24:35–63.
Kelly LM, Gilliland DG. Genetics of myeloid leukemias. Annu Rev Genomics Hum Genet. 2002;3:179–98.
Yan X-J, Xu J, Gu Z-H, Pan C-M, Lu G, Shen Y, et al. Exome sequencing identifies somatic mutations of DNA methyltransferase gene DNMT3A in acute monocytic leukemia. Nat Genet [Internet]. 2011;43:309–15. http://www.nature.com/doifinder/10.1038/ng.788.
Yamaguchi S, Iwanaga E, Tokunaga K, Nanri T, Shimomura T, Suzushima H, et al. IDH1 and IDH2 mutations confer an adverse effect in patients with acute myeloid leukemia lacking the NPM1 mutation. Eur J Haematol. 2014;92:471–7.
Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood [Internet]. 2016;127:2391–405. http://www.ncbi.nlm.nih.gov/pubmed/27069254.
Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, Vardiman J, editors. WHO classification of tumours of haematopoietic and lymphoid tissues. 4a ed. Lyon: International Agency for Research on Cancer (IARC); 2008.
Stirewalt DL, Radich JP. The role of FLT3 in haematopoietic malignancies. Nat Rev Cancer [Internet]. 2003 [cited 2014 Jul 16];3:650–65. http://www.ncbi.nlm.nih.gov/pubmed/12951584.
Abu-Duhier FM, Goodeve AC, Wilson GA, Care RS, Peake IR, Reilly JT. Genomic structure of human FLT3: implications for mutational analysis. Br J Haematol [Internet]. 2001 [cited 2015 Jan 13];113:1076–7. http://doi.wiley.com/10.1046/j.1365-2141.2001.02821.x.
Schmidt-Arras DE, Bohmer A, Markova B, Choudhary C, Serve H, Bohmer FD. Tyrosine phosphorylation regulates maturation of receptor tyrosine kinases. Mol Cell Biol [Internet]. 2005;25:3690–703. http://mcb.asm.org/cgi/doi/10.1128/MCB.25.9.3690-3703.2005.
Rosnet O, Schiff C, Pébusque MJ, Marchetto S, Tonnelle C, Toiron Y, et al. Human FLT3/FLK2 gene: cDNA cloning and expression in hematopoietic cells. Blood [Internet]. 1993;82:1110–9. http://www.ncbi.nlm.nih.gov/pubmed/8394751.
Lyman SD, Jacobsen SE. c-kit ligand and Flt3 ligand: stem/progenitor cell factors with overlapping yet distinct activities. Blood [Internet]. 1998 [cited 2014 Oct 22];91:1101–34. http://bloodjournal.hematologylibrary.org/content/91/4/1101.short.
Waskow C, Liu K, Darrasse-Jèze G, Guermonprez P, Ginhoux F, Merad M, et al. The receptor tyrosine kinase Flt3 is required for dendritic cell development in peripheral lymphoid tissues. Nat Immunol [Internet]. 2008;9:676–83. http://www.nature.com/doifinder/10.1038/ni.1615.
Griffith J, Black J, Faerman C, Swenson L, Wynn M, Lu F, et al. the structural basis for autoinhibition of FLT3 by the juxtamembrane domain. Mol Cell [Internet]. 2004 [cited 2014 Nov 4];13:169–78. http://linkinghub.elsevier.com/retrieve/pii/S1097276503005057.
Ke Y-Y, Singh VK, Coumar MS, Hsu YC, Wang W-C, Song J-S, et al. Homology modeling of DFG-in FMS-like tyrosine kinase 3 (FLT3) and structure-based virtual screening for inhibitor identification. Sci Rep [Internet]. 2015;5:11702. http://www.nature.com/doifinder/10.1038/srep11702.
Rocnik JL. Roles of tyrosine 589 and 591 in STAT5 activation and transformation mediated by FLT3–ITD. Blood [Internet]. 2006;108:1339–45. http://www.bloodjournal.org/cgi/doi/10.1182/blood-2005-11-011429.
Verstraete K, Vandriessche G, Januar M, Elegheert J, Shkumatov A V, Desfosses A, et al. Structural insights into the extracellular assembly of the hematopoietic Flt3 signaling complex. Blood [Internet]. 2011 [cited 2014 Aug 29];118:60–8. http://www.ncbi.nlm.nih.gov/pubmed/21389326.
Turner AM, Lin NL, Issarachai S, Lyman SD, Broudy VC. FLT3 receptor expression on the surface of normal and malignant human hematopoietic cells. Blood [Internet]. 1996;88:3383–90. http://www.ncbi.nlm.nih.gov/pubmed/8896403.
Nakao M, Yokota S, Iwai T, Kaneko H, Horiike S, Kashima K, et al. Internal tandem duplication of the flt3 gene found in acute myeloid leukemia. Leukemia [Internet]. 1996 [cited 2014 Oct 31];10:1911–8. http://www.ncbi.nlm.nih.gov/pubmed/8946930.
Janke H, Pastore F, Schumacher D, Herold T, Hopfner K-P, Schneider S, et al. Activating FLT3 mutants show distinct gain-of-function phenotypes in vitro and a characteristic signaling pathway profile associated with prognosis in acute myeloid leukemia. PLoS ONE [Internet]. 2014;9:e89560. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3946485&tool=pmcentrez&rendertype=abstract.
Mead AJ, Linch DC, Hills RK, Wheatley K, Burnett AK, Gale RE. FLT3 tyrosine kinase domain mutations are biologically distinct from and have a significantly more favorable prognosis than FLT3 internal tandem duplications in patients with acute myeloid leukemia. Blood. 2007;110:1262–70.
Sandhöfer N, Bauer J, Reiter K, Dufour A, Rothenberg M, Konstandin NP, et al. The new and recurrent FLT3 juxtamembrane deletion mutation shows a dominant negative effect on the wild-type FLT3 receptor. Sci Rep [Internet]. The Author(s); 2016;6:28032. http://dx.doi.org/10.1038/srep28032.
Reindl C, Bagrintseva K, Vempati S, Schnittger S, Ellwart JW, Wenig K, et al. Point mutations in the juxtamembrane domain of FLT3 define a new class of activating mutations in AML. Blood [Internet]. 2006;107:3700–7. http://www.bloodjournal.org/cgi/doi/10.1182/blood-2005-06-2596.
Schittenhelm MM, Yee KWH, Tyner JW, McGreevey L, Haley a D, Town a, et al. FLT3 K663Q is a novel AML-associated oncogenic kinase: determination of biochemical properties and sensitivity to Sunitinib (SU11248). Leuk Off J Leuk Soc Am Leuk Res Fund, U.K. 2006;20:2008–14.
Kiyoi H, Ohno R, Ueda R, Saito H, Naoe T. Mechanism of constitutive activation of FLT3 with internal tandem duplication in the juxtamembrane domain. Oncogene [Internet]. 2002 [cited 2014 Aug 16];21:2555–63. http://europepmc.org/abstract/MED/11971190.
Chatain N, Perera RC, Rossetti G, Rossa J, Carloni P, Schemionek M, et al. Rare FLT3 deletion mutants may provide additional treatment options to patients with AML: an approach to individualized medicine. Leukemia [Internet]. 2015;1–4. http://www.nature.com/doifinder/10.1038/leu.2015.131.
Levis M, Small D. FLT3: ITDoes matter in leukemia. Leukemia [Internet]. 2003 [cited 2014 Oct 17];17:1738–52. http://www.ncbi.nlm.nih.gov/pubmed/12970773.
Meshinchi S, Appelbaum FR. Structural and functional alterations of FLT3 in acute myeloid leukemia. Clin Cancer Res. 2009;15:4263–9.
Kayser S, Schlenk RF, Londono MC, Breitenbuecher F, Wittke K, Du J, et al. Insertion of FLT3 internal tandem duplication in the tyrosine kinase domain-1 is associated with resistance to chemotherapy and inferior outcome. Blood. 2009;114:2386–92.
Kiyoi H, Towatari M, Yokota S, Hamaguchi M, Ohno R, Saito H, et al. Internal tandem duplication of the FLT3 gene is a novel modality of elongation mutation which causes constitutive activation of the product. Leukemia [Internet]. 1998 [cited 2014 Oct 14];12:1333–7. http://www.nature.com/leu/journal/v12/n9/pdf/2401130a.pdf.
Chia W, Savakis C, Karp R, Pelham H, Ashburner M. Mutation of the Adh gene of Drosophila-melanogaster containing an internal tandem duplication. J Mol Biol. 1985;186:679–88.
Corbacioglu S, Kilic M, Westhoff M-A, Reinhardt D, Fulda S, Debatin K-M. Newly identified c-KIT receptor tyrosine kinase ITD in childhood AML induces ligand-independent growth and is responsive to a synergistic effect of imatinib and rapamycin. Blood [Internet]. 2006;108:3504–13. http://www.bloodjournal.org/cgi/doi/10.1182/blood-2006-05-021691.
Webster JD, Yuzbasiyan-Gurkan V, Kaneene JB, Miller R, Resau JH, Kiupel M. The role of c-KIT in tumorigenesis: evaluation in canine cutaneous mast cell tumors. Neoplasia [Internet]. 2006;8:104–11. http://linkinghub.elsevier.com/retrieve/pii/S1476558606800199.
Astolfi A, Melchionda F, Perotti D, Fois M, Indio V, Urbini M, et al. Whole transcriptome sequencing identifies BCOR internal tandem duplication as a common feature of clear cell sarcoma of the kidney. Oncotarget [Internet]. 2015;6. http://www.oncotarget.com/abstract/5882.
Meshinchi S, Stirewalt DL, Alonzo TA, Boggon TJ, Gerbing RB, Rocnik JL, et al. Structural and numerical variation of FLT3/ITD in pediatric AML. Blood [Internet]. 2008 [cited 2014 Aug 29];111:4930–3. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2384125&tool=pmcentrez&rendertype=abstract.
Porter SN, Cluster AS, Yang W, Busken KA, Patel RM, Ryoo J, et al. Fetal and neonatal hematopoietic progenitors are functionally and transcriptionally resistant to FLT3– ITD mutations. Elife [Internet]. 2016;5:e18882. http://elifesciences.org/lookup/doi/10.7554/eLife.18882.
Zwaan CM, Meshinchi S, Radich JP, Veerman AJP, Huismans DR, Munske L, et al. FLT3 internal tandem duplication in 234 children with acute myeloid leukemia: prognostic significance and relation to cellular drug resistance. Blood [Internet]. 2003;102:2387–94. http://www.ncbi.nlm.nih.gov/pubmed/12816873.
Meshinchi S, Alonzo TA, Stirewalt DL, Zwaan M, Zimmerman M, Reinhardt D, et al. Clinical implications of FLT3 mutations in pediatric AML. Blood [Internet]. 2006 [cited 2015 Jan 26];108:3654–61. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1895470&tool=pmcentrez&rendertype=abstract.
Schnittger S, Schoch C, Dugas M, Kern W, Staib P, Wuchter C, et al. Analysis of FLT3 length mutations in 1003 patients with acute myeloid leukemia: correlation to cytogenetics, FAB subtype, and prognosis in the AMLCG study and usefulness as a marker for the detection of minimal residual disease. Blood [Internet]. 2002 [cited 2014 Oct 22];100:59–66. http://www.ncbi.nlm.nih.gov/pubmed/12070009.
Steudel C, Wermke M, Schaich M, Schäkel U, Illmer T, Ehninger G, et al. Comparative analysis of MLL partial tandem duplication and FLT3 internal tandem duplication mutations in 956 adult patients with acute myeloid leukemia. Genes Chromosom Cancer [Internet]. 2003;37:237–51. http://doi.wiley.com/10.1002/gcc.10219.
Li L, Piloto O, Kim KT, Ye Z, Ho BN, Yu X, et al. FLT3/ITD expression increases expansion, survival and entry into cell cycle of human haematopoietic stem/progenitor cells. Br J Haematol [Internet]. 2007 [cited 2014 Oct 22];137:64–75. http://www.ncbi.nlm.nih.gov/pubmed/17359372.
Li L, Piloto O, Nguyen HB, Greenberg K, Takamiya K, Racke F, et al. Knock-in of an internal tandem duplication mutation into murine FLT3 confers myeloproliferative disease in a mouse model. Blood [Internet]. 2008;111:3849–58. http://www.bloodjournal.org/cgi/doi/10.1182/blood-2007-08-109942.
Lee BH, Williams IR, Anastasiadou E, Boulton CL, Joseph SW, Amaral SM, et al. FLT3 internal tandem duplication mutations induce myeloproliferative or lymphoid disease in a transgenic mouse model. Oncogene [Internet]. 2005;24:7882–92. http://www.nature.com/doifinder/10.1038/sj.onc.1208933.
Vardiman JW, Harris NL, Brunning RD. The World Health Organization (WHO) classification of the myeloid neoplasms. Blood [Internet]. 2002 [cited 2014 Oct 24];100:2292–302. http://www.ncbi.nlm.nih.gov/pubmed/12239137.
Thiede C, Steudel C, Mohr B, Schaich M, Schäkel U, Platzbecker U, et al. Analysis of FLT3-activating mutations in 979 patients with acute myelogenous leukemia: association with FAB subtypes and identification of subgroups with poor prognosis. Blood [Internet]. 2002;99:4326–35. http://www.ncbi.nlm.nih.gov/pubmed/12036858.
Kottaridis PD, Gale RE, Frew ME, Harrison G, Langabeer SE, Belton AA, et al. The presence of a FLT3 internal tandem duplication in patients with acute myeloid leukemia (AML) adds important prognostic information to cytogenetic risk group and response to the first cycle of chemotherapy: analysis of 854 patients from the United King. Blood [Internet]. 2001;98:1752–9. http://www.ncbi.nlm.nih.gov/pubmed/11535508.
Patel JP, Gönen M, Figueroa ME, Fernandez H, Sun Z, Racevskis J, et al. Prognostic relevance of integrated genetic profiling in acute myeloid leukemia. N Engl J Med [Internet]. 2012;366:1079–89. http://www.nejm.org/doi/abs/10.1056/NEJMoa1112304.
Papaemmanuil E, Gerstung M, Bullinger L, Gaidzik VI, Paschka P, Roberts ND, et al. Genomic classification and prognosis in acute myeloid leukemia. N Engl J Med. [Internet]. 2016;374:2209–21. http://www.nejm.org/doi/10.1056/NEJMoa1516192.
Chan PM. Differential signaling of Flt3 activating mutations in acute myeloid leukemia: a working model. Protein Cell [Internet]. 2011 [cited 2014 Sep 28];2:108–15. http://www.ncbi.nlm.nih.gov/pubmed/21359601.
Choudhary C, Olsen J V, Brandts C, Cox J, Reddy PNG, Böhmer FD, et al. Mislocalized activation of oncogenic RTKs switches downstream signaling outcomes. Mol Cell [Internet]. 2009 [cited 2014 Aug 13];36:326–39. http://www.ncbi.nlm.nih.gov/pubmed/19854140.
Hayakawa F, Towatari M, Kiyoi H, Tanimoto M, Kitamura T, Saito H, et al. Tandem-duplicated Flt3 constitutively activates STAT5 and MAP kinase and introduces autonomous cell growth in IL-3-dependent cell lines. Oncogene [Internet]. 2000 [cited 2015 Jan 20];19:624–31. http://www.ncbi.nlm.nih.gov/pubmed/10698507.
Okamoto M, Hayakawa F, Miyata Y, Watamoto K, Emi N, Abe A, et al. Lyn is an important component of the signal transduction pathway specific to FLT3/ITD and can be a therapeutic target in the treatment of AML with FLT3/ITD. Leukemia [Internet]. 2007 [cited 2014 Aug 29];21:403–10. http://www.ncbi.nlm.nih.gov/pubmed/17230226.
Leischner H, Albers C, Grundler R, Razumovskaya E, Spiekermann K, Bohlander S, et al. SRC is a signaling mediator in FLT3–ITD- but not in FLT3–TKD–positive AML. Blood [Internet]. 2012 [cited 2014 Oct 22];119:4026–33. http://www.ncbi.nlm.nih.gov/pubmed/22411868.
Park JE, Yuen HF, Zhou JB, Al-aidaroos AQO, Guo K, Valk PJ, et al. Oncogenic roles of PRL-3 in FLT3–ITD induced acute myeloid leukaemia. EMBO Mol Med. 2013;5:1351–66.
Nabinger SC, Li XJ, Ramdas B, He Y, Zhang X, Zeng L, et al. The protein tyrosine phosphatase, Shp2, positively contributes to FLT3–ITD-induced hematopoietic progenitor hyperproliferation and malignant disease in vivo. Leukemia [Internet]. 2013;27:398–408. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3916934&tool=pmcentrez&rendertype=abstract.
Pillinger G, Abdul-Aziz A, Zaitseva L, Lawes M, MacEwan DJ, Bowles KM, et al. Targeting BTK for the treatment of FLT3–ITD mutated acute myeloid leukemia. Sci Rep [Internet]. Nature Publishing Group; 2015;5:12949. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=4544001&tool=pmcentrez&rendertype=abstract.
Wu M, Hamaker M, Li L, Small D, Duffield AS. DOCK2 interacts with FLT3 and modulates the survival of FLT3-expressing leukemia cells. Leukemia [Internet]. 2017;31:688–96. http://www.nature.com/doifinder/10.1038/leu.2016.284.
Caldarelli a, Müller JP, Paskowski-Rogacz M, Herrmann K, Bauer R, Koch S, et al. A genome-wide RNAi screen identifies proteins modulating aberrant FLT3–ITD signaling. Leukemia [Internet]. 2013 [cited 2015 Jan 23];27:2301–10. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3865536&tool=pmcentrez&rendertype=abstract.
Schmidt-Arras D, Bohmer S-A, Koch S, Muller JP, Blei L, Cornils H, et al. Anchoring of FLT3 in the endoplasmic reticulum alters signaling quality. Blood [Internet]. 2009;113:3568–76. http://www.bloodjournal.org/cgi/doi/10.1182/blood-2007-10-121426.
Mead A, Kharazi S, Atkinson D, Macaulay I, Pecquet C, Loughran S, et al. FLT3–ITDs instruct a myeloid differentiation and transformation bias in lymphomyeloid multipotent progenitors. Cell Rep [Internet]. The Authors; 2013;3:1766–76. http://dx.doi.org/10.1016/j.celrep.2013.04.031.
Li L, Bailey E, Greenblatt S, Huso D, Small D. Loss of the wild-type allele contributes to myeloid expansion and disease aggressiveness in FLT3/ITD knockin mice. Blood [Internet]. 2011 [cited 2014 Nov 2];118:4935–45. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3208299&tool=pmcentrez&rendertype=abstract.
Mizuki M, Schwable J, Steur C, Choudhary C, Agrawal S, Sargin B, et al. Suppression of myeloid transcription factors and induction of STAT response genes by AML-specific Flt3 mutations. Blood [Internet]. 2003;101:3164–73. http://www.bloodjournal.org/cgi/doi/10.1182/blood-2002-06-1677.
Choudhary C. AML-associated Flt3 kinase domain mutations show signal transduction differences compared with Flt3 ITD mutations. Blood [Internet]. 2005;106:265–73. http://www.bloodjournal.org/cgi/doi/10.1182/blood-2004-07-2942.
Yang L, Rodriguez B, Mayle A, Park HJ, Lin X, Luo M, et al. DNMT3A loss drives enhancer hypomethylation in FLT3–ITD-associated leukemias. Cancer Cell [Internet]. 2016;30:363–5. http://linkinghub.elsevier.com/retrieve/pii/S153561081630349X.
Sallmyr A, Fan J, Datta K, Kim K-T, Grosu D, Shapiro P, et al. Internal tandem duplication of FLT3 (FLT3/ITD) induces increased ROS production, DNA damage, and misrepair: implications for poor prognosis in AML. Blood [Internet]. 2008;111:3173–82. http://www.bloodjournal.org/cgi/doi/10.1182/blood-2007-05-092510.
Stanicka J, Russell EG, Woolley JF, Cotter TG. NADPH oxidase-generated hydrogen peroxide induces DNA damage in mutant FLT3-expressing Leukemia Cells. J Biol Chem [Internet]. 2015;290:9348–61. http://www.jbc.org/lookup/doi/10.1074/jbc.M113.510495.
Kottaridis PD, Gale RE, Linch DC. FLT3 mutations and leukaemia. Br J Haematol. 2003;122:523–38.
Schnittger S, Bacher U, Kern W, Alpermann T, Haferlach C, Haferlach T. Prognostic impact of FLT3–ITD load in NPM1 mutated acute myeloid leukemia. Leukemia [Internet]. Nature Publishing Group; 2011 [cited 2015 Jan 23];25:1297–304. http://www.ncbi.nlm.nih.gov/pubmed/21537333.
Gale RE, Green C, Allen C, Mead AJ, Burnett AK, Hills RK, et al. The impact of FLT3 internal tandem duplication mutant level, number, size, and interaction with NPM1 mutations in a large cohort of young adult patients with acute myeloid leukemia. Blood [Internet]. 2008 [cited 2014 Nov 6];111:2776–84. http://www.ncbi.nlm.nih.gov/pubmed/17957027.
Schlenk RF, Kayser S, Bullinger L, Kobbe G, Casper J, Ringhoffer M, et al. Differential impact of allelic ratio and insertion site in FLT3–ITD-positive AML with respect to allogeneic transplantation. Blood [Internet]. 2014;124:3441–9. http://www.bloodjournal.org/cgi/doi/10.1182/blood-2014-05-578070.
Blau O, Berenstein R, Sindram A, Blau IW. Molecular analysis of different FLT3–ITD mutations in acute myeloid leukemia. Leuk Lymphoma [Internet]. 2013 [cited 2015 Jan 23];54:145–52. http://www.ncbi.nlm.nih.gov/pubmed/22721497.
Wu X, Feng X, Zhao X, Ma F, Liu N, Guo H, et al. Prognostic significance of FLT3–ITD in pediatric acute myeloid leukemia: a meta-analysis of cohort studies. Mol Cell Biochem [Internet]. Springer US; 2016;420:121–8. http://link.springer.com/10.1007/s11010-016-2775-1.
Stirewalt DL, Pogosova-Agadjanyan EL, Tsuchiya K, Joaquin J, Meshinchi S. Copy-neutral loss of heterozygosity is prevalent and a late event in the pathogenesis of FLT3/ITD AML. Blood Cancer J [Internet]. Nature Publishing Group; 2014 [cited 2014 Oct 23];4:e208. http://dx.doi.org/10.1038/bcj.2014.27.
Whitman SP, Archer KJ, Feng L, Baldus C, Becknell B, Carlson BD, et al. Absence of the wild-type allele predicts poor prognosis in adult de novo acute myeloid leukemia with normal cytogenetics and the internal tandem duplication of FLT3: a cancer and leukemia group B study. Cancer Res [Internet]. 2001;61:7233–9. http://www.ncbi.nlm.nih.gov/pubmed/11585760.
Stirewalt DL. Size of FLT3 internal tandem duplication has prognostic significance in patients with acute myeloid leukemia. Blood [Internet]. 2006 [cited 2014 Aug 29];107:3724–6. http://bloodjournal.hematologylibrary.org/content/107/9/3724.short.
Chillón MC, Santamaría C, García-Sanz R, Balanzategui A, Sarasquete ME, Alcoceba M, et al. Long FLT3 internal tandem duplications and reduced PML-RAR?? expression at diagnosis characterize a high-risk subgroup of acute promyelocytic leukemia patients. Haematologica. 2010;95:745–51.
Ponziani V, Gianfaldoni G, Mannelli F, Leoni F, Ciolli S, Guglielmelli P, et al. The size of duplication does not add to the prognostic significance of FLT3 internal tandem duplication in acute myeloid leukemia patients. Leukemia [Internet]. 2006 [cited 2014 Aug 29];20:2074–6. http://www.ncbi.nlm.nih.gov/pubmed/16990788.
Schnittger S, Bacher U, Haferlach C, Alpermann T, Kern W, Haferlach T. Diversity of the juxtamembrane and TKD1 mutations (Exons 13–15) in the FLT3 gene with regards to mutant load, sequence, length, localization, and correlation with biological data. Genes Chromosom Cancer [Internet]. 2012;51:910–24. http://doi.wiley.com/10.1002/gcc.21975.
Kusec R. More on prognostic significance of FLT3/ITD size in acute myeloid leukemia (AML). Blood [Internet]. 2006 [cited 2015 Jan 23];108:405–6. http://www.ncbi.nlm.nih.gov/pubmed/16790588.
Arreba-Tutusaus P, Mack TS, Bullinger L, Schnöder TM, Polanetzki A, Weinert S, et al. Impact of FLT3–ITD location on sensitivity to TKI-therapy in vitro and in vivo. Leukemia [Internet]. 2016;30:1220–5. http://www.nature.com/doifinder/10.1038/leu.2015.292.
Cloos J, Goemans BF, Hess CJ, van Oostveen JW, Waisfisz Q, Corthals S, et al. Stability and prognostic influence of FLT3 mutations in paired initial and relapsed AML samples. Leukemia [Internet]. 2006 [cited 2014 Aug 29];20:1217–20. http://www.ncbi.nlm.nih.gov/pubmed/16642044.
Zuffa E, Franchini E, Papayannidis C, Baldazzi C, Simonetti G, Testoni N, et al. Revealing very small FLT3 ITD mutated clones by ultra-deep sequencing analysis has important clinical implications in AML patients. Oncotarget [Internet]. 2015;6:31284–94. http://www.ncbi.nlm.nih.gov/pubmed/26384303.
Chou W-C, Hou H-A, Liu C-Y, Chen C-Y, Lin L-I, Huang Y-N, et al. Sensitive measurement of quantity dynamics of FLT3 internal tandem duplication at early time points provides prognostic information. Ann Oncol [Internet]. 2011;22:696–704. http://annonc.oxfordjournals.org/cgi/doi/10.1093/annonc/mdq402.
Kottaridis PD, Gale RE, Langabeer SE, Frew ME, Bowen DT, Linch DC. Studies of FLT3 mutations in paired presentation and relapse samples from patients with acute myeloid leukemia: implications for the role of FLT3 mutations in leukemogenesis, minimal residual disease detection, and possible therapy with FLT3 inhibitors. Blood. 2002;100:2393–8.
Berman E, Maloy M, Devlin S, Jhanwar S, Papadopoulos E, Jakubowski A. Stem cell transplantation in adults with acute myelogenous leukemia, normal cytogenetics, and the FLT3–ITD mutation. Leuk Res [Internet]. Elsevier Ltd; 2016;40:33–7. http://linkinghub.elsevier.com/retrieve/pii/S014521261530549X.
Libura M, Asnafi V, Tu A, Delabesse E, Tigaud I, Cymbalista F, et al. FLT3 and MLL intragenic abnormalities in AML reflect a common category of genotoxic stress. Blood. 2003;102:2198–204.
Levis M. FLT3 mutations in acute myeloid leukemia: what is the best approach in 2013? Hematol. Am. Soc. Hematol. Educ. Program [Internet]. 2013 [cited 2014 Nov 5];2013:220–6. http://www.ncbi.nlm.nih.gov/pubmed/24319184.
Beretta C, Gaipa G, Rossi V, Bernasconi S, Spinelli O, Dell’Oro MG, et al. Development of a quantitative-PCR method for specific FLT3/ITD monitoring in acute myeloid leukemia. Leukemia [Internet]. 2004 [cited 2015 Jan 23];18:1441–4. http://www.ncbi.nlm.nih.gov/pubmed/15201851.
Beierl K, Tseng L-H, Beierl R, Haley L, Gocke CD, Eshleman JR, et al. Detection of minor clones with internal tandem duplication mutations of FLT3 gene in acute myeloid leukemia using delta-PCR. Diagn Mol Pathol Am J Surg Pathol Part B [Internet]. 2013;22:1–9. http://www.ncbi.nlm.nih.gov/pubmed/23370424.
Lin M-T, Tseng L-H, Beierl K, Hsieh A, Thiess M, Chase N, et al. Tandem duplication PCR: an ultra-sensitive assay for the detection of internal tandem duplications of the FLT3 gene. Diagn Mol Pathol [Internet]. 2013;22:149–55. http://content.wkhealth.com/linkback/openurl?sid=WKPTLP:landingpage&an=00019606-201309000-00004.
Gari M, Abuzenadah A, Chaudhary A, Al-Qahtani M, Banni H, Ahmad W, et al. Detection of FLT3 oncogene mutations in acute myeloid leukemia using conformation sensitive gel electrophoresis. Int J Mol Sci [Internet]. 2008 [cited 2014 Aug 29];9:2194–204. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2635624&tool=pmcentrez&rendertype=abstract.
Bianchini M, Ottaviani E, Grafone T, Giannini B, Soverini S, Terragna C, et al. Rapid detection of Flt3 mutations in acute myeloid leukemia patients by denaturing HPLC. Clin Chem [Internet]. 2003;49:1642–50. http://www.ncbi.nlm.nih.gov/pubmed/14500589.
Shouval R, Shlush LI, Yehudai-Resheff S, Ali S, Pery N, Shapiro E, et al. Single cell analysis exposes intratumor heterogeneity and suggests that FLT3–ITD is a late event in leukemogenesis. Exp Hematol [Internet]. ISEH—Society for Hematology and Stem Cells; 2014;42:457–63. http://dx.doi.org/10.1016/j.exphem.2014.01.010.
Murphy KM, Levis M, Hafez MJ, Geiger T, Cooper LC, Smith BD, et al. Detection of FLT3 internal tandem duplication and D835 mutations by a multiplex polymerase chain reaction and capillary electrophoresis assay. J Mol Diagn. 2003;5:96–102.
Spencer DH, Abel HJ, Lockwood CM, Payton JE, Szankasi P, Kelley TW, et al. Detection of FLT3 internal tandem duplication in targeted, short-read-length, next-generation sequencing data. J Mol Diagn [Internet]. American Society for Investigative Pathology and the Association for Molecular Pathology; 2013;15:81–93. http://dx.doi.org/10.1016/j.jmoldx.2012.08.001.
Angiolini M. Targeting the DFG-in kinase conformation: a new trend emerging from a patent analysis. Future Med Chem [Internet]. 2011;3:309–37. http://www.future-science.com/doi/10.4155/fmc.10.294.
Grunwald MR, Levis MJ. FLT3 inhibitors for acute myeloid leukemia: a review of their efficacy and mechanisms of resistance. Int J Hematol [Internet]. 2013 [cited 2015 Jan 23];97:683–94. http://www.ncbi.nlm.nih.gov/pubmed/23613268.
Chu SH, Small D. Mechanisms of resistance to FLT3 inhibitors. Drug Resist Updat. 2009;12:8–16.
Heidel F. Clinical resistance to the kinase inhibitor PKC412 in acute myeloid leukemia by mutation of Asn-676 in the FLT3 tyrosine kinase domain. Blood [Internet]. 2006;107:293–300. http://www.bloodjournal.org/cgi/doi/10.1182/blood-2005-06-2469.
Williams AB, Nguyen B, Li L, Brown P, Levis M, Leahy D, et al. Mutations of FLT3/ITD confer resistance to multiple tyrosine kinase inhibitors. Leukemia [Internet]. 2013 [cited 2015 Jan 9];27:48–55. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3822911&tool=pmcentrez&rendertype=abstract.
Albers C, Leischner H, Verbeek M, Yu C, Illert a L, Peschel C, et al. The secondary FLT3–ITD F691L mutation induces resistance to AC220 in FLT3–ITD + AML but retains in vitro sensitivity to PKC412 and Sunitinib. Leukemia [Internet]. 2013;27:1416–8. http://www.ncbi.nlm.nih.gov/pubmed/23392356.
Cools J, Mentens N, Furet P, Fabbro D, Clark JJ, Griffin JD, et al. Prediction of resistance to small molecule FLT3 inhibitors: implications for molecularly targeted therapy of acute leukemia. Cancer Res [Internet]. 2004;64:6385–9. http://cancerres.aacrjournals.org/cgi/doi/10.1158/0008-5472.CAN-04-2148.
Smith CC, Wang Q, Chin C-S, Salerno S, Damon LE, Levis MJ, et al. Validation of ITD mutations in FLT3 as a therapeutic target in human acute myeloid leukaemia. Nature [Internet]. Nature Publishing Group; 2012;485:260–3. http://dx.doi.org/10.1038/nature11016.
Bagrintseva K, Geisenhof S, Kern R, Eichenlaub S, Reindl C, Ellwart JW, et al. FLT3–ITD-TKD dual mutants associated with AML confer resistance to FLT3 PTK inhibitors and cytotoxic agents by overexpression of Bcl-x(L). Blood [Internet]. 2005;105:3679–85. http://www.bloodjournal.org/cgi/doi/10.1182/blood-2004-06-2459.
Luzzatto L. Acquired resistance to imatinib mesylate: selection for pre-existing mutant cells. Blood [Internet]. 2002;100:1105–6. http://www.bloodjournal.org/cgi/doi/10.1182/blood-2002-05-1578.
Leung a YH, Man C-H, Kwong Y-L. FLT3 inhibition: a moving and evolving target in acute myeloid leukaemia. Leukemia [Internet]. Nature Publishing Group; 2012;27:260–8. http://dx.doi.org/10.1038/leu.2012.195.
Reddy PNG, Sargin B, Choudhary C, Stein S, Grez M, Müller-Tidow C, et al. SOCS1 cooperates with FLT3-ITD in the development of myeloproliferative disease by promoting the escape from external cytokine control. Blood [Internet]. 2012;120:1691–702. http://www.ncbi.nlm.nih.gov/pubmed/22517899.
Perner F, Schnöder TM, Fischer T, Heidel FH. Kinomics screening identifies aberrant phosphorylation of CDC25C in FLT3-ITD-positive AML. Anticancer Res [Internet]. 2016;36:6249–58. http://ar.iiarjournals.org/content/36/12/6249.abstract.
Metzelder SK, Michel C, von Bonin M, Rehberger M, Hessmann E, Inselmann S, et al. NFATc1 as a therapeutic target in FLT3-ITD-positive AML. Leukemia [Internet]. 2015;29:1470–7. http://www.nature.com/doifinder/10.1038/leu.2015.95.
Hirade T, Abe M, Onishi C, Taketani T, Yamaguchi S, Fukuda S. Internal tandem duplication of FLT3 deregulates proliferation and differentiation and confers resistance to the FLT3 inhibitor AC220 by Up-regulating RUNX1 expression in hematopoietic cells. Int J Hematol [Internet]. 2016;103:95–106. http://link.springer.com/10.1007/s12185-015-1908-8.
Damdinsuren A, Matsushita H, Ito M, Tanaka M, Jin G, Tsukamoto H, et al. FLT3-ITD drives Ara-C resistance in leukemic cells via the induction of RUNX3. Leuk Res [Internet]. 2015;39:1405–13. http://dx.doi.org/10.1016/j.leukres.2015.09.009.
Yoshimoto G, Miyamoto T, Jabbarzadeh-Tabrizi S, Iino T, Rocnik JL, Kikushige Y, et al. FLT3-ITD up-regulates MCL-1 to promote survival of stem cells in acute myeloid leukemia via FLT3-ITD-specific STAT5 activation. Blood [Internet]. 2009 [cited 2014 Oct 22];114:5034–43. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2788977&tool=pmcentrez&rendertype=abstract.
Kim K-T. Pim-1 is up-regulated by constitutively activated FLT3 and plays a role in FLT3-mediated cell survival. Blood [Internet]. 2005;105:1759–67. http://www.bloodjournal.org/cgi/doi/10.1182/blood-2004-05-2006.
Green AS, Maciel TT, Hospital M-A, Yin C, Mazed F, Townsend EC, et al. Pim kinases modulate resistance to FLT3 tyrosine kinase inhibitors in FLT3-ITD acute myeloid leukemia. Sci Adv [Internet]. 2015;1:e1500221. http://advances.sciencemag.org/cgi/doi/10.1126/sciadv.1500221.
Puissant A, Fenouille N, Alexe G, Pikman Y, Bassil CF, Mehta S, et al. SYK is a critical regulator of FLT3 in acute myeloid leukemia. Cancer Cell [Internet]. 2014;25:226–42. http://www.ncbi.nlm.nih.gov/pubmed/24525236.
Hattori A, McSkimming D, Kannan N, Ito T. RNA binding protein MSI2 positively regulates FLT3 expression in myeloid leukemia. Leuk Res [Internet]. 2017;54:47–54. http://linkinghub.elsevier.com/retrieve/pii/S0145212617300152.
Kazi JU, Rönnstrand L. Suppressor of cytokine signaling 2 (SOCS2) associates with FLT3 and negatively regulates downstream signaling. Mol Oncol [Internet]. 2013;7:693–703. http://doi.wiley.com/10.1016/j.molonc.2013.02.020.
Kazi JU, Sun J, Phung B, Zadjali F, Flores-Morales A, Ronnstrand L. Suppressor of cytokine signaling 6 (SOCS6) negatively regulates FLT3 signal transduction through direct binding to phosphorylated tyrosines 591 and 919 of FLT3. J Biol Chem [Internet]. 2012;287:36509–17. http://www.jbc.org/cgi/doi/10.1074/jbc.M112.376111.
Scheijen B, Ngo HT, Kang H, Griffin JD. FLT3 receptors with internal tandem duplications promote cell viability and proliferation by signaling through Foxo proteins. Oncogene [Internet]. 2004 [cited 2014 Oct 13];23:3338–49. http://www.ncbi.nlm.nih.gov/pubmed/14981546.
Lin D-C, Yin T, Koren-Michowitz M, Ding L-W, Gueller S, Gery S, et al. Adaptor protein Lnk binds to and inhibits normal and leukemic FLT3. Blood [Internet]. 2012 [cited 2015 Jan 26];120:3310–7. http://www.bloodjournal.org/cgi/doi/10.1182/blood-2011-10-388611.
Moharram SA, Chougule RA, Su X, Li T, Sun J, Zhao H, et al. Src-like adaptor protein 2 (SLAP2) binds to and inhibits FLT3 signaling. Oncotarget [Internet]. 2016;7. http://www.ncbi.nlm.nih.gov/pubmed/27458164.
Kazi JU, Vaapil M, Agarwal S, Bracco E, Påhlman S, Rönnstrand L. The tyrosine kinase CSK associates with FLT3 and c-Kit receptors and regulates downstream signaling. Cell Signal. [Internet]. 2013;25:1852–60. http://dx.doi.org/10.1016/j.cellsig.2013.05.016.
Kazi JU, Rupar K, Marhäll A, Moharram SA, Khanum F, Shah K, et al. ABL2 suppresses FLT3-ITD-induced cell proliferation through negative regulation of AKT signaling. Oncotarget [Internet]. 2017. http://www.oncotarget.com/abstract/14577.
Chen P, Levis M, Brown P, Kim K-T, Allebach J, Small D. FLT3/ITD mutation signaling includes suppression of SHP-1. J Biol Chem [Internet]. 2005;280:5361–9. http://www.jbc.org/cgi/doi/10.1074/jbc.M411974200.
Al-Mawali A, Gillis D, Lewis I. Immunoprofiling of leukemic stem cells CD34+/CD38−/CD123 + delineate FLT3/ITD-positive clones. J Hematol Oncol [Internet]. J Hemat Oncol; 2016;9:61. http://jhoonline.biomedcentral.com/articles/10.1186/s13045-016-0292-z.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Lagunas-Rangel, F.A., Chávez-Valencia, V. FLT3–ITD and its current role in acute myeloid leukaemia. Med Oncol 34, 114 (2017). https://doi.org/10.1007/s12032-017-0970-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-017-0970-x