Abstract
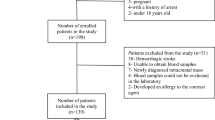
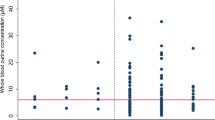
Fast diagnosis and appropriate treatment are of utmost importance to improving the outcome in patients with acute ischemic stroke (AIS). A rapid and sensitive blood test for ischemic stroke is required. The aim of this study was to examine the usefulness of phenylalanine (PHE) and tyrosine (TYR) as diagnostic biomarkers in AIS. Serum levels of PHE and TYR, measured using HPLC, and their ratio (PHE/TYR) were compared between 45 patients with AIS and 40 healthy control subjects. The relationship between PHE/TYR and the serum levels of several cytokines were also examined. PHE/TYR was significantly higher in AIS patients than in healthy controls (1.75 vs 1.24, p < 0.001). A receiver operating characteristic (ROC) curve analysis of PHE/TYR in AIS patients relative to healthy controls revealed promising sensitivity and specificity, which at an optimal cutoff of 1.45 were 76 and 85 %, respectively. PHE/TYR was positively correlated with interleukin (IL)-1β (r = 0.37, p = 0.011) and IL-6 (r = 0.33, p = 0.025). This study shows that PHE/TYR is highly elevated in the acute phase of AIS, and that this elevation is coupled to the inflammatory response. The ROC analysis documents the possible value of PHE/TYR as a biomarker for AIS and demonstrates its clinical potential as a blood-based test for AIS.




Similar content being viewed by others
References
Adams HP Jr et al (1993) Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke 24:35–41
Anderson DN et al (1994) Recovery from depression after electroconvulsive therapy is accompanied by evidence of increased tetrahydrobiopterin-dependent hydroxylation. Acta Psychiatr Scand 90:10–13
Askanazi J et al (1980) Muscle and plasma amino acids following injury. Influence of intercurrent infection. Ann Surg 192:78–85
Brouns R, De Deyn PP (2009) The complexity of neurobiological processes in acute ischemic stroke. Clin Neurol Neurosurg 111:483–495
Chiarla C, Giovannini I, Siegel JH (2004) The relationship between plasma cholesterol, amino acids and acute phase proteins in sepsis. Amino Acids 27:97–100
Collin C et al (1988) The Barthel ADL Index: a reliability study. Int Disabil Stud 10:61–63
Crabtree MJ, Channon KM (2011) Synthesis and recycling of tetrahydrobiopterin in endothelial function and vascular disease. Nitric Oxide 25:81–88
Crabtree MJ, Hale AB, Channon KM (2011) Dihydrofolate reductase protects endothelial nitric oxide synthase from uncoupling in tetrahydrobiopterin deficiency. Free Radic Biol Med 50:1639–1646
Foerch C et al (2009) Invited article: searching for oracles? Blood biomarkers in acute stroke. Neurology 73:393–399
Forstermann U, Sessa WC (2012) Nitric oxide synthases: regulation and function. Eur Heart J 33:829–837, 837a-837d
Freund H et al (1979) Plasma amino acids as predictors of the severity and outcome of sepsis. Ann Surg 190:571–576
Fuchs JE et al (2012) Dynamic regulation of phenylalanine hydroxylase by simulated redox manipulation. PLoS One 7:e53005
Hasan N et al (2012) Towards the identification of blood biomarkers for acute stroke in humans: a comprehensive systematic review. Br J Clin Pharmacol 74:230–240
Hoekstra R et al (2001) Effect of electroconvulsive therapy on biopterin and large neutral amino acids in severe, medication-resistant depression. Psychiatry Res 103:115–123
Jin R, Yang G, Li G (2010) Inflammatory mechanisms in ischemic stroke: role of inflammatory cells. J Leukoc Biol 87:779–789
Kim SJ, Moon GJ, Bang OY (2013) Biomarkers for stroke. J Stroke 15:27–37
Kwon NS, Nathan CF, Stuehr DJ (1989) Reduced biopterin as a cofactor in the generation of nitrogen oxides by murine macrophages. J Biol Chem 264:20496–20501
Lerdal A, et al. 2012. The course of fatigue during the first 18 months after first-ever stroke: a longitudinal study. Stroke research and treatment. 2012, 126275
Lopansri BK et al (2006) Elevated plasma phenylalanine in severe malaria and implications for pathophysiology of neurological complications. Infect Immun 74:3355–3359
Love S (1999) Oxidative stress in brain ischemia. Brain Pathol 9:119–131
Manzanero S, Santro T, Arumugam TV (2013) Neuronal oxidative stress in acute ischemic stroke: sources and contribution to cell injury. Neurochem Int 62:712–718
McColl BW, Allan SM, Rothwell NJ (2009) Systemic infection, inflammation and acute ischemic stroke. Neuroscience 158:1049–1061
Nanetti L et al (2007) Reactive oxygen species plasmatic levels in ischemic stroke. Mol Cell Biochem 303:19–25
Neurauter G et al (2008a) Serum phenylalanine concentrations in patients with ovarian carcinoma correlate with concentrations of immune activation markers and of isoprostane-8. Cancer Lett 272:141–147
Neurauter G et al (2008b) Chronic immune stimulation correlates with reduced phenylalanine turnover. Curr Drug Metab 9:622–627
Ormstad H et al (2011a) Serum cytokine and glucose levels as predictors of poststroke fatigue in acute ischemic stroke patients. J Neurol 258:670–676
Ormstad H et al (2011b) Serum levels of cytokines and C-reactive protein in acute ischemic stroke patients, and their relationship to stroke lateralization, type, and infarct volume. J Neurol 258:677–685
Ploder M et al (2008) Serum phenylalanine in patients post trauma and with sepsis correlate to neopterin concentrations. Amino Acids 35:303–307
Powanda MC et al (1974) Distribution and metabolism of phenylalanine and tyrosine during tualraemia in the rat. Biochem J 144:173–176
Ribas GS et al (2011) Oxidative stress in phenylketonuria: what is the evidence? Cell Mol Neurobiol 31:653–662
Rosenblatt D, Scriver CR (1968) Heterogeneity in genetic control of phenylalanine metabolism in man. Nature 218:677–678
Rothstein L, Jickling GC (2013) Ischemic stroke biomarkers in blood. Biomark Med 7:37–47
Schmidt TS, Alp NJ (2007) Mechanisms for the role of tetrahydrobiopterin in endothelial function and vascular disease. Clin Sci (Lond) 113:47–63
Sprung CL et al (1991) Amino acid alterations and encephalopathy in the sepsis syndrome. Crit Care Med 19:753–757
Taffi R et al (2008) Plasma levels of nitric oxide and stroke outcome. J Neurol 255:94–98
Turnell DC, Cooper JD (1982) Rapid assay for amino acids in serum or urine by pre-column derivatization and reversed-phase liquid chromatography. Clin Chem 28:527–531
Vente JP et al (1989) Plasma-amino acid profiles in sepsis and stress. Ann Surg 209:57–62
Vogelgesang A, Becker KJ, Dressel A (2014) Immunological consequences of ischemic stroke. Acta Neurol Scand 129:1–12
Wannemacher RW Jr et al (1976) The significance and mechanism of an increased serum phenylalanine-tyrosine ratio during infection. Am J Clin Nutr 29:997–1006
Werner ER, Blau N, Thony B (2011) Tetrahydrobiopterin: biochemistry and pathophysiology. Biochem J 438:397–414
Whiteley W, Tseng MC, Sandercock P (2008) Blood biomarkers in the diagnosis of ischemic stroke: a systematic review. Stroke 39:2902–2909
Whiteley W, Tian Y, Jickling GC (2012) Blood biomarkers in stroke: research and clinical practice. Int J Stroke 7:435–439
Whitsett J et al (2013) Human endothelial dihydrofolate reductase low activity limits vascular tetrahydrobiopterin recycling. Free Radic Biol Med 63:143–150
Wolfe CD et al (1991) Assessment of scales of disability and handicap for stroke patients. Stroke 22:1242–1244
Acknowledgments
The authors are indebted to the various staff members of Vestre Viken Hospital Trust, Buskerud, Drammen, and Oslo University Hospital, Ullevål, Oslo, for important contributions to the study. The presented work stems from the research project “Poststroke Fatigue,” for which Dr. Hesook Suzie Kim is the project director and Drs. Grethe Eilertsen, Anners Lerdal, and Heidi Ormstad are the principal researchers. The project is funded by the Research Council of Norway for the period from 2007 to 2010 (project no. 176503/V10), and Vestre Viken Hospital Trust.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The study was approved by The Regional Committee for Medical Research Ethics in Norway and has therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki. Informed consent was obtained from all patients prior to their inclusion in the study.
Conflict of Interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Ormstad, H., Verkerk, R. & Sandvik, L. Serum Phenylalanine, Tyrosine, and their Ratio in Acute Ischemic Stroke: on the Trail of a Biomarker?. J Mol Neurosci 58, 102–108 (2016). https://doi.org/10.1007/s12031-015-0659-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-015-0659-6