Abstract
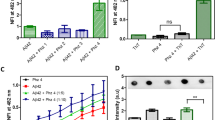
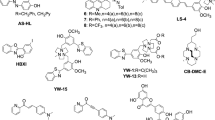
Alzheimer’s disease is the most common cause of dementia in older individuals with compelling evidence favoring neuron dysfunction and death triggered by assembled forms of Aβ1–42. While large neurotoxic amyloid fibrils have been known for years, recent studies show that soluble protofibril and Aβ1–42-derived diffusible ligands (ADDLs) may also be involved in neurotoxicity. In the present work, dot-blot immunoassays discriminating ADDLs from monomers were used to screen libraries of per-substituted β-cyclodextrin (β-CD) derivatives for inhibition of ADDLs formation. Libraries were prepared from per-6-iodo-β-CD by treatment with various amine nucleophiles. The most active library tested (containing >2000 derivatives) was derived from imidazole, N, N-dimethylethylenediamine and furfurylamine, which at 10 µM total library, inhibited ADDLs formation (10 nM Aβ1–42) over a period of 4 hours. The latter was confirmed by a western blot assay showing decreased amounts of the initially formed Aβ1–42 tetramer. These preliminary experiments suggest that derivatized forms of β-CD can interfere with the oligomerization process of Aβ1–42.
Similar content being viewed by others
References
Ashton P. R., Koniger R., Stoddart J. F., Alker D., and Harding V. D. (1996) Amino acid derivatives of beta-cyclodextrin. J. Org. Chem. 61, 903–908.
Camilleri P., Haskins J. J., and Howlett D. R. (1994) beta-Cyclodextrin interacts with the Alzheimer amyloid beta-A4 peptide. Fed. Euro. Biochem. Soc. Lett. 341, 256–258.
Drouet B., Pincon-Raymond M., and Chambaz J, Pillot T. (1999) Laminin 1 attenuates beta-amyloid peptide Abeta (1–40) neurotoxicity of cultured fetal rat cortical neurons. J. Neurochem. 73, 742–749.
Givol D. (1974) Affinity labeling and topology of the antibody combining site. Essays Biochem. 10, 73–103.
Golde T. E., Eckman C. B., and Younkin S. G. (2000) Biochemical detection of Abeta isoforms: implications for pathogenesis, diagnosis, and treatment of Alzheimer’s disease. J. Biochem. Biophys. Acta 1502, 172–187.
Hartley D. M., Walsh D. M., Ye C. P., Diehl T., Vasquez S., Vassilev P. M., et al. (1999) Profibrillar intermediates of amyloid beta-protein induce acute electrophysiological changes and progressive neurotoxicity in cortical neurons. J. Neurosci. 19, 8876–8884.
Howlett D., Cutler P., Heales S., and Camilleri P. (1997) Hemin and related porphyrins inhibit beta-amyloid aggregation. Fed. Euro. Biochem. Soc. Lett. 417, 249–251.
Howlett D. R., Perry A. E., Godfrey F., Swatton J. E., Jennings K. H., Spitzfaden C., et al. (1999) Inhibition of fibril formation in beta-amyloid peptide by a novel series of benzofurans. Biochem. J. 340, 283–289.
Hsia A. Y., Masliah E., McConlogue I., Yu G. Q., Tatsuno G., Hu K., et al. (1999) Plaque-independent disruption of neural circuits in Alzheimer’s disease mouse models. Proc. Natl. Acad. Sci. USA 96, 3228–3233.
Klein W. L. (2001) Fibrils, Protofibrils & Abeta-Derived Diffusible Ligands: How Abeta Causes Neuron Dysfunction and Death in Alzheimer’s Disease. Humana Press, Totowa, NJ.
Lambert M. P., Barlow A. K., Chromy B. A., Edwards C., Freed R., Liosatos M., et al. (1998) Diffusible, nonfibrillar ligands derived from Abeta1-42 are potent central nervous system neurotoxins. Proc. Natl. Acad. Sci. USA 95, 6448–6453.
Lambert M. P., Viola K. L., Cromy B. A., Chang L., Morgan T. E., Yu J., et al. (2001) Vaccination with soluble Abeta oligomers generates toxicity-neutralizing antibodies. J. Neurochem., 79, 595–605.
Longo V. D., Viola K. L., Klein W. L., and Finch C. E. (2000) Reversible inactivation of superoxide-sensitive aconitase in Abeta1-42-treated neuronal cell lines. J. Neurochem. 75, 12243–12247.
Lorenzo A. and Yankner B. A. (1994) Beta-amyloid neurotoxicity requires fibril formation and is inhibited by Congo red. Proc. Natl. Acad. Sci. USA 91, 12243–12247.
Mucke L., Masliah E., Yu G. Q., Mallory M., Rockenstein E. M., Tatsuno G., et al. (2000) High-level neuronal expression of Abeta 1–42 in wild-type human amy-loid protein precursor transgenic mice: synaptotoxicity without plaque formation. J. Neurosci. 20, 4050–4058.
Pappolla M., Bozner P., Soto C., Shao H., Robakis N. K., Zagorski M., Frangione B., and Ghiso J. (1998) Inhibition of Alzheimer beta-fibrillogenesis by melatonin. J. Biol. Chem. 273, 7185–7188.
Pike C. J., Burdick D., Walencewicz A. J., Glabe C. G., Cotman C. W. (1993) Neurodegeneration induced by beta-amyloid peptides in vitro: the role of peptide assembly state. J. Neurosci. 13, 1676–1687.
Schubert D. (1997) Serpins inhibit the toxicity of amyloid peptides. Eur. J. Neurosci. 9, 770–777.
Small D.H. (1998) The amyloid cascade hypothesis debate: emerging consensus on the role of Abeta and amyloid in Alzheimer’s disease. The Sixth International Conference on Alzheimer’s disease, Amsterdam, The Netherlands, pp. 301–304.
Solomon B., Koppel R., Frankel D., and Hanan-Aharon E. (1997) Disaggregation of Alzheimer beta-amyloid by site-directed mAb. Proc. Natl. Acad. Sci. USA 94, 4109–4112.
Solomon B., Koppel R., Hanan E., and Katzav T. (1996) Monoclonal antibodies inhibit in vitro fibrillar aggregation of the Alzheimer beta-amyloid peptide. Proc. Natl. Acad. Sci. USA 93, 452–455.
Soto C., Kindy M. S., Baumann M., and Frangione B. (1996) Inhibition of Alzheimer’s amyloidosis by peptides that prevent beta-sheet conformation. Biochem. Biophys. Res. Comm. 226, 672–680.
Soto C., Sigurdsson E. M., Morelli L., Kumar R. A., Castano E. M., and Frangione B. (1998) Beta-sheet breaker peptides inhibit fibrillogenesis in a rat brain model of amyloidosis: implications for Alzheimer’s therapy [see comments]. Nature Med. 4, 822–826.
Tomiyama T., Asano S., Suwa Y., Morita T., Kataoka K., Mori H., and Endo N. (1994) Rifampicin prevents the aggregation and neurotoxicity of amyloid beta protein in vitro. Biochem. Biophys. Res. Commun. 204, 76–83.
Tomiyama T., Shoji A., Kataoka K., Suwa Y., Asano S., Kaneko H., and Endo N. (1996) Inhibition of amyloid beta protein aggregation and neurotoxicity by rifampicin. Its possible function as a hydroxyl radical scavenger. J. Biol. Chem. 271, 6839–6844.
Waite J., Cole G. M., Frautschy S. A., Connor D. J., and Thal L. J. (1992) Solvent effects on beta protein toxicity in vivo. Neurobiol. Aging 13, 595–599.
Walsh D. M., Hartley D. M., Kusumoto Y., Fezoui Y., Condron M. M., Lomakin A., et al. (1999) Amyloid beta-protein fibrillogenesis. Structure and biological activity of protofibrillar intermediates. J. Biol. Chem. 274, 25945–25952.
Walsh D. M., Lomakin A., Benedek G. B., Condron M. M., and Teplow D. B. (1997) Amyloid beta-protein fibrillogenesis. Detection of a protofibrillar intermediate. J. Biol. Chem. 272, 22364–22372.
Wood S. J., MacKenzie L., Maleff B., Hurle M. R., and Wetzel R. (1996) Selective inhibition of Abeta fibril formation. J. Biol. Chem. 271, 4086–4092.
Yu G. S., Hu J., and Nakagawa H. (1998) Inhibition of beta-amyloid cytotoxicity by midkine. Neurosci. Lett. 254, 125–128.
Yu J., Zhao Y., Holterman M. J., and Venton D. L. (1999) Combinatorial search of substituted beta-cyclodextrins for phosphatase-like activity. Bioorg. Med. Chem. Lett. 9, 2705–2710.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yu, J., Bakhos, L., Chang, L. et al. Per-6-substituted β-cyclodextrin libraries inhibit formation of β-amyloid-peptide (Aβ)-derived, soluble oligomers. J Mol Neurosci 19, 51–55 (2002). https://doi.org/10.1007/s12031-002-0010-x
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s12031-002-0010-x