Abstract
Purpose
Molecular analysis of peritoneal fluid in staging laparoscopy of gastric cancer is performed to improve the detection of free intraperitoneal tumor cells. Nevertheless, its significance is controversial, especially in patients with negative cytology but positive molecular analysis.
The aim of this study was to analyze the sensitivity of molecular analysis and its prognostic value.
Methods
A retrospective analysis from April 2011 to October 2019 was performed. Cytology (Cyt) and molecular analysis were analyzed by real-time reverse transcriptase polymerase chain reaction (RT-PCR) of the carcinoembryonic antigen (CEA) and cytokeratin 20 (CK20) tumor makers.
Results
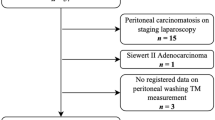
During the study period, 138 staging laparoscopies were performed. Macroscopic carcinomatosis was found in 12.3%. Of the remaining 87.7%, 9.9% were Cyt + and 11.6% were Cyt- RT-PCR + . Of the latter, 9 responded to chemotherapy and underwent radical surgery. The sensitivity of cytology and molecular analysis was 0.70 and 0.76, respectively (p = 0.67).
The 2-year overall survival (OS) of Cyt- RT-PCR + vs. Cyt + was similar (p = 0.1). The 2-year OS of Cyt-RT-PCR + subgroup who underwent radical surgery vs. Cyt-RT-PCR- patients was similar (p = 0.69), but disease-free survival was shorter in the first group (p = 0.005).
Conclusion
Our results show that the sensitivity of molecular analysis is similar to that of cytology. The prognostic value of positive molecular analysis was similar to positive cytology in terms of 2-year overall survival, except in the subgroup of operated patients in whom the overall survival was similar to that of those with a negative molecular analysis, albeit with a shorter disease-free survival.






Similar content being viewed by others
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. https://doi.org/10.3322/caac.21492.
Ajani J, D’Amico T, Almhanna K, Bentrem DJ, Chao J, Das P, et al. Gastric cancer, version 3. 2016, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2016;14:1286–1312. https://doi.org/10.6004/jnccn.2016.0137
Smyth EC, Verheij M, AllumW, Cunningham D, Cervantes A, Arnold D, ESMO Guidelines Committee. Gastric cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2016;27(suppl 5):v38-v49. https://doi.org/10.1093/annonc/mdw350
Waddell T, Verheij M, Allum W, Cunningham D, Cervante A, Arnold D, European Society for Medical Oncology (ESMO), European Society of Surgical Oncology (ESSO), European Society of Radiotherapy and Oncology (ESTRO). Gastric cancer: ESMO-ESSO-ESTRO clinical guidelines for diagnosis, treatment and follow-up. Ann Oncol 2013;24(suppl 6):vi 57–63. https://doi.org/10.1093/annonc/mdt344
Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2018 (5th edition). Gastric Cancer 2021;24:1–21. https://doi.org/10.1007/s10120-020-01042-y
De Andrade J, Mezhir JJ. The critical role of peritoneal cytology in the staging of gastric cancer: an evidence-based review. J Surg Oncol. 2014;110;291–297. https://doi.org/10.1002/jso.23632
Frattini F, Rausei S, Chiappa C, Rovera F, Boni L, Dionigi G. Prognosis and treatment of patients with positive peritoneal citology in advanced gastric cancer. World J Gastrointest Surg. 2013;27:135–7. https://doi.org/10.4240/wjgs.v5.i5.135.
Taffon C, Giovannoni I, Mozetic P, Capolupo GT, La Vaccara V, Cinque C, et al. Seriate cytology vs molecular analysis of peritoneal washing to improve gastric cancer cells detection. Diagn Cytopathol. 2019;47:670–4. https://doi.org/10.1002/dc.24165.
Wong J, Kelly KJ, Mittra A, Gonen M, Allen P, Fong Y, et al. RT-PCR increase detection of submicroscopic peritoneal metastases in gastric cancer and has pronostic significance. J Gastrointest Surg. 2012;16:889–896. https://doi.org/10.1007/s11605-012-1845-2
Dalal KM, Woo Y, Kelly K, Galanis C, Gonen M, Fong Y, et al. Detection of micrometastases in peritoneal washings of gastric cancer patients by the reverse transcripatse polymerase chain reaction. Gastric Cancer. 2008;11:206–13. https://doi.org/10.1007/s10120-008-0483-6.
Wang JY, Lin SR, Lu CY, Chen CC, Wu DC, Chai CY et al. Gastric cancer cell detection in peritoneal lavage: RT-PCR for carcinoembryonic antigen transcripts versus combined cytology with peritoneal carcinoembryonic antigen levels. Cancer Lett. 2005;223:129–35. https://doi.org/10.1016/j.canlet.2004.09.031
Deng, K, Zhu H, Chen M, Wu J, Hu R, Tang C. Prognostic significance of molecular analysis of peritonela fluid for patients with gastric cancer: a meta-analysis. PloS One 2016;17:1–22. https://doi.org/10.1371/journal.pone.0151608
Tamura S, Fujiwara Y, Kimura Y, et al. Prognostic information derived from RT-PCR analysis of peritoneal fluid in gastric cancer patients: results from a prospective multicenter clinical trial. J Surg Oncol. 2014;109:75–80. https://doi.org/10.1002/jso.23472.
Machairas N, Charalampoudis P, Molmenti E, Kykalos S, Tsparas P, Stamopoulos P, et al. The value of staging laparoscopy in gastric cancer. Annals of Gastroenterology 2017;30:287–294. https://doi.org/10.20524/aog.2017.0133.
Kodera Y, Nakanishi H, Ito S, Yamamura Y, Kanemitsu Y, Shimizu Y, et al. Quantitative detection of diseminated free cancer cells in peritoneal washes with real-time reverse transcriptase-polymerase chain reactin: a sensitive predictor of outcome for patients with gastric carcinoma. Ann Surg. 2002;235:499–506. https://doi.org/10.1097/00000658-200204000-00007.
Edge S, Byrd D, Compton C, Fritz A, Greene F, Trotti A. AJCC cancer staging manual. 7th ed. New York: Springer; 2010. p. 117–21.
Saklad M. Grading of patients for surgical procedures. Anesthesiol. 1941;2:281–4. https://doi.org/10.1097/00000542-194105000-00004.
Dindo D, Demartines N, Clavien PA. Classification of surgical complications. A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–13. https://doi.org/10.1097/01.sla.0000133083.54934.ae.
Fujiwara Y, Okada K, Hanada H, Tamura S, Kimura Y, Fujita J, et al. The clinical importance of a transcription reverse-transcription concerted (TRC) diagnosis using peritoneal lavage fluids in gastric cancer with clinical serosal invasion: a prospective multicente study. Surgery. 2014;155:417–23. https://doi.org/10.1016/j.surg.2013.10.004.
Kodera Y; Nakanishi H, Ito S, Mochizuki Y, Ohashi N, Yammaura Y et al. Prognostic significance on intrapeirotneal cancer cells in gastric carcinoma: analysis of real time reverse transcriptase-polymerase chain reaction after 5 years of followup. J AM Coll Surg. 2006;202:231–236. https://doi.org/10.1016/j.jamcollsurg.2005.09.008.
Fujiwara Y, Doki Y, Taniguchi H, Sohma I, Takiguchi S, Miyata H, et al. Genetic detection of free cancer cells in the peritoneal cavity of the patient with gastric cancer: present status and future perspectives. Gastric Cancer 2007;10:197–204. https://doi.org/10.1007/s10120-007-0436-5.
Xiao Y, Zhang X, He X, Ji J, Wang G. Diagnostic values of carcinoembryonic antigen in predicting peritoenal recurrence after curative resection of gastric cancer: a meta-analysis. Ir J Med Sci. 2013;183:557–564. https://doi.org/10.1007/s11845-013-1051-6.
Virgilio E, Giarnieri E, Giovagnoli MR, et al. Gastric cancer cells in peritoneal lavage fluid: a systematic review comparing cytological with molecular detection for diagnosis of peritoneal metastases and prediction of peritoneal recurrences. Anticancer Res. 2018;38: 1255–1262. https://doi.org/10.21873/anticanres.12347
Kagawa S, Shigeyasu S, Ishida M, Watanabe M, Tazawa H, Nagasaka T, Shirakawa Y, Fujiwara T. Molecular diagnosis and therapy for occult peritoneal metastasis in gastric cancer patients World J Gastroenterol 2014;20(47):17796-17803 https://doi.org/10.3748/wjg.v20.i47.17796
Li Z, Zhang D, Zhang H, Miao Z, Tang Y, Sun G and Dai D. Prediction of peritoneal recurrence by the mRNA level of CEA and MMP-7 in peritoneal lavage of gastric cancer patients. Tumor Biol. 2014;35:3463–3470. https://doi.org/10.1007/s13277-013-1458-8.
Pecqueux M, Fritzmann J, Adamu M, Thorlund K, Kahlert C, Reißfelder C, et al. Free peritoneal tumor cells and outcome in gastric cancer patients: a systematic review and meta-analysis. Oncotarget 2015;34:35564–35578. https://doi.org/10.18632/oncotarget.5595.
Matsuoka T, Yashiro M. Significance of peritoneal lavage cytology based on genetic signatures in gastric cancer. J J Cancer Metastasis Treat 2018;4:6;1–13. https://doi.org/10.20517/2394-4722.2017.85
Nakanishi K, Kanda M, Umeda S, Tanaka C, Kobayashi D, Hayashi M et al. The levels of SYT13 and CEA mRNAs in peritoneal lavages predict the peritoneal recurrence of gastric cancer. Gastric cancer 2019;22(6):1143–1152. https://doi.org/10.1007/s10120-019-00967-3.
Armstrong DK, Bundy B, Wenzel L, et al. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl J Med. 2006;354:34–43. https://doi.org/10.1056/NEJMoa052985.
Yonemura Y, Endou Y, Bando E, et al. Effect of intraperitoneal administration of docetaxel on peritoneal dissemination of gastric cancer. Cancer Lett. 2004;210:189–96. https://doi.org/10.1016/j.canlet.2004.03.018.
Tamura S, Miki H, Okada K, et al. Pilot study of intraperitoneal administration of paclitaxel and oral S.1 for patients with peritoneal metastasis due to advanced gastric cancer. Int J Clin Oncol. 2008;13:536–40. https://doi.org/10.1007/s10147-008-0836-5.
Hamazoe R, Maeta M, Kaibara N. Intraperitoneal thermochemo therapy for prevention of peritoneal recurrence of gastric cancer. Final results of randomized controlled study. Cancer 1994;73: 2048–2052. https://pubmed.ncbi.nlm.nih.gov/8156509/
Fujiwara Y, Takiguchi S, Nakajima K, et al. Intraperitoneal docetaxel combined with S.1 for advanced gastric cancer with peritoneal dissemination. J Surg Oncol. 2011;105:38–42. https://doi.org/10.1002/jso.22057
Acknowledgements
The authors thank D. Pringle for reviewing the final draft of the manuscript for its content and English style. The authors would like to thank the IDIBELL Foundation for the institutional support provided.
Funding
This study was supported by the Institut d’Investigació Biomèdica de Bellvitge (IDIBELL Foundation) (structural funding).
Author information
Authors and Affiliations
Contributions
Miró M: protocol, data collection, data analysis, manuscript writing. Vives R: data collection, manuscript writing. Farran L: protocol, final revision. Secanella L: protocol, data analysis. Varela M and Baixeras N: cytology and molecular analysis, manuscript writing. Estremiana F, Bettonica C and Aranda H: protocol, data collection, final revision. Galán M: protocol, final revision. All authors approved the submitted version.
Corresponding author
Ethics declarations
Ethics approval
Written informed consent was considered not necessary for the study as it was a retrospective analysis of our usual everyday work. Data of patients were anonymized for the purposes of this analysis. The confidential information of the patients was protected according to European regulations. This manuscript has been revised for its publication by the Research Ethics Committee of Bellvitge University Hospital.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Miró, M., Vives, R., Farran, L. et al. Utility of Molecular Analysis of Peritoneal Fluid in Staging Laparoscopy of Advanced Esophagogastric Junction and Gastric Cancer Prior to Neoadjuvant Treatment. J Gastrointest Canc 54, 651–661 (2023). https://doi.org/10.1007/s12029-022-00846-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12029-022-00846-8