Abstract
Purpose
To investigate the prognostic effects of baseline volumetric PET/CT parameters including the maximum standard uptake value (SUVmax), metabolic tumor volume (MTV), and tumor lesion glycolysis (TLG) on treatment response and prognosis in locally advanced rectal cancer (LARC) treated with neoadjuvant chemoradiotherapy (NACRT).
Methods
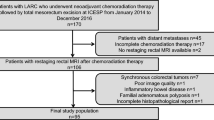
Between 2015 and 2018, 51 patients with LARC treated with NACRT followed by surgery were included in this retrospective study. Patients were divided into 2 groups by tumor regression grade (TRG) as follows: group I = TRG 1 (no detectable cancer cells) + TRG 2 (single cells and/or small groups of cancer cells) and group II = TRG3 (residual tumor outgrown by fibrosis) + TRG 4 (remarkable fibrosis outgrown by tumor cells) + TRG 5 (no fibrosis with extensive residual cancer).
Results
Of the 51 patients, 34 (66.7%) were male. The median age was 55 (range, 37–78) years. According to TRG status, 14 (27.4%) patients were in group I and 37 (72.6%) patients were in group II. The area under the curve (95% CI) was 0.749 (0.593–0.905) in the ROC curve plotted for MTV. The cut-off value for MTV was 12, with 70% sensitivity and 65% specificity. MTV was ≥ 12 in 32 (62.8%) patients. MTV and TLG values were significantly different between groups I and II, whereas there was no significant difference between the groups in terms of SUVmax values (p = 0.006, p = 0.033, and p = 0.673, respectively). The disease-free survival was not reached in patients with MTV < 12 vs. 20 months in those with MTV ≥ 12 (p = 0.323). In multivariate analysis, MTV (OR, 95% Cl, 5.00 [1.17–21.383]) was found to be the factor that affected pathological complete response.
Conclusion
In LARC treated with NACRT, MTV prior to treatment can help predict the response to treatment.


Similar content being viewed by others
References
Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471–4.
Heald RJ. The “Holy Plane” of rectal surgery. J R Soc Med. 1988;81:503–8.
Li Y, Wang J, Ma X, et al. A review of neoadjuvant chemoradiotherapy for locally advanced rectal cancer. Int J Biol Sci. 2016;12:1022–31.
Martin ST, Heneghan HM, Winter DC. Systematic review and meta-analysis of outcomes following pathological complete response to neoadjuvant chemoradiotherapy for rectal cancer. Br J Surg. 2012;99:918–28.
Bujko K, Kolodziejczyk M, Nasierowska-Guttmejer A, et al. Tumour regression grading in patients with residual rectal cancer after preoperative chemoradiation. Radiother Oncol. 2010;95:298–302.
Topova L, Hellmich G, Puffer E, et al. Prognostic value of tumor response to neoadjuvant therapy in rectal carcinoma. Dis Colon Rectum. 2011;54:401–11.
Shivnani AT, Small W Jr, Stryker SJ, et al. Preoperative chemoradiation for rectal cancer: results of multimodality management and analysis of prognostic factors. Am J Surg. 2007;193:389–93.
Habr-Gama A, Perez RO, Nadalin W, et al. Operative versus nonoperative treatment for stage 0 distal rectal cancer following chemoradiation therapy: long-term results. Ann Surg. 2004;240:711–7.
Maas M, Nelemans PJ, Valentini V, et al. Long-term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: a pooled analysis of individual patient data. Lancet Oncol. 2010;11:835–44.
Sakin A, Sahin S, Sengul Samanci N, et al. The impact of tumor regression grade on long-term survival in locally advanced rectal cancer treated with preoperative chemoradiotherapy. J Oncol Pharm Pract. 2020;1078155219900944.
Paun BC, Cassie S, MacLean AR, et al. Postoperative complications following surgery for rectal cancer. Ann Surg. 2010;251:807–18.
van der Valk MJM, Hilling DE, Bastiaannet E, et al. Long-term outcomes of clinical complete responders after neoadjuvant treatment for rectal cancer in the International Watch & Wait Database (IWWD): an international multicentre registry study. Lancet. 2018;391:2537–45.
Appelt AL, Ploen J, Harling H, et al. High-dose chemoradiotherapy and watchful waiting for distal rectal cancer: a prospective observational study. Lancet Oncol. 2015;16:919–27.
Rombouts AJM, Al-Najami I, Abbott NL, et al. Can we Save the rectum by watchful waiting or TransAnal microsurgery following (chemo) Radiotherapy versus Total mesorectal excision for early REctal Cancer (STAR-TREC study)?: protocol for a multicentre, randomised feasibility study. BMJ Open. 2017;7:e019474.
Gallamini A, Zwarthoed C, Borra A. Positron emission tomography (PET) in oncology. Cancers (Basel). 2014;6:1821–89.
Boellaard R, Delgado-Bolton R, Oyen WJ, et al. FDG PET/CT: EANM procedure guidelines for tumour imaging: version 2.0. Eur J Nucl Med Mol Imaging. 2015;42:328–54.
Liao S, Penney BC, Wroblewski K, et al. Prognostic value of metabolic tumor burden on 18F-FDG PET in nonsurgical patients with non-small cell lung cancer. Eur J Nucl Med Mol Imaging. 2012;39:27–38.
Song BI, Kim HW, Won KS, et al. Preoperative standardized uptake value of metastatic lymph nodes measured by 18F-FDG PET/CT improves the prediction of prognosis in gastric cancer. Medicine (Baltimore). 2015;94:e1037.
Arici S, Karyagar SS, Karyagar S, et al. The predictive role of metabolic tumor volume on no response to neoadjuvant chemotherapy in patients with breast cancer. J Oncol Pharm Pract. 2020,1078155219898504.
Husby JA, Reitan BC, Biermann M, et al. Metabolic tumor volume on 18F-FDG PET/CT improves preoperative identification of high-risk endometrial carcinoma patients. J Nucl Med. 2015;56:1191–8.
Li QW, Zheng RL, Ling YH, et al. Prediction of tumor response after neoadjuvant chemoradiotherapy in rectal cancer using (18)fluorine-2-deoxy-D-glucose positron emission tomography-computed tomography and serum carcinoembryonic antigen: a prospective study. Abdom Radiol (NY). 2016;41:1448–55.
Pellegrino S, Fonti R, Mazziotti E, et al. Total metabolic tumor volume by 18F-FDG PET/CT for the prediction of outcome in patients with non-small cell lung cancer. Ann Nucl Med. 2019;33:937–44.
Ryan R, Gibbons D, Hyland JM, et al. Pathological response following long-course neoadjuvant chemoradiotherapy for locally advanced rectal cancer. Histopathology. 2005;47:141–6.
Chen CC, Lee RC, Lin JK, et al. How accurate is magnetic resonance imaging in restaging rectal cancer in patients receiving preoperative combined chemoradiotherapy? Dis Colon Rectum. 2005;48:722–8.
Rau B, Hunerbein M, Barth C, et al. Accuracy of endorectal ultrasound after preoperative radiochemotherapy in locally advanced rectal cancer. Surg Endosc. 1999;13:980–4.
Calvo FA, Domper M, Matute R, et al. 18F-FDG positron emission tomography staging and restaging in rectal cancer treated with preoperative chemoradiation. Int J Radiat Oncol Biol Phys. 2004;58:528–35.
Mac Manus MP, Hicks RJ, Matthews JP, et al. Positron emission tomography is superior to computed tomography scanning for response-assessment after radical radiotherapy or chemoradiotherapy in patients with non-small-cell lung cancer. J Clin Oncol. 2003;21:1285–92.
Martoni AA, Zamagni C, Quercia S, et al. Early (18)F-2-fluoro-2-deoxy-d-glucose positron emission tomography may identify a subset of patients with estrogen receptor-positive breast cancer who will not respond optimally to preoperative chemotherapy. Cancer. 2010;116:805–13.
Gauthe M, Richard-Molard M, Fayard J, et al. Prognostic impact of tumour burden assessed by metabolic tumour volume on FDG PET/CT in anal canal cancer. Eur J Nucl Med Mol Imaging. 2017;44:63–70.
Lee SJ, Kim JG, Lee SW, et al. Clinical implications of initial FDG-PET/CT in locally advanced rectal cancer treated with neoadjuvant chemoradiotherapy. Cancer Chemother Pharmacol. 2013;71:1201–7.
Jo HJ, Kim SJ, Lee HY, et al. Prediction of survival and cancer recurrence using metabolic volumetric parameters measured by 18F-FDG PET/CT in patients with surgically resected rectal cancer. Clin Nucl Med. 2014;39:493–7.
Jo HJ, Kim SJ, Kim IJ, et al. Predictive value of volumetric parameters measured by F-18 FDG PET/CT for lymph node status in patients with surgically resected rectal cancer. Ann Nucl Med. 2014;28:196–202.
Kim SH, Song BI, Kim BW, et al. Predictive value of [(18)F]FDG PET/CT for lymph node metastasis in rectal cancer. Sci Rep. 2019;9:4979.
Beddy D, Hyland JM, Winter DC, et al. A simplified tumor regression grade correlates with survival in locally advanced rectal carcinoma treated with neoadjuvant chemoradiotherapy. Ann Surg Oncol. 2008;15:3471–7.
Author information
Authors and Affiliations
Contributions
Concept-AS, SS, SSK, SC; design-AS, MMS, MHA, SC; supervision-SS, SK, MMA, SC; resources-AS, SSK, SK, MMA; materials-SK, MHA, MMA, SC; data collection and/or processing-AS, SS, SK, MHA; analysis and/or interpretation-SS, SSK, MMK, SC; literature search-AS, SS, MMA, SC; writing manuscript-AS, SS, SSK, SK; critical review-SS, SC; other-AS, SS, SSK, SK.
Corresponding author
Ethics declarations
Institutional Review Board Statement
All necessary procedures for this study were implemented based on the declaration of Helsinki. The ethics committee approval was taken from the Ethics Committee Board of University of Health Sciences Okmeydani Training and Research Hospital (ID: 48670771-514.10).
Informed Consent
The patients were not required to give informed consent for this study because the study utilized the anonymous retrospective data obtained after each patient accepted the treatment by a written consent.
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sakin, A., Sahin, S., Karyagar, S.S. et al. The Predictive Value of Baseline Volumetric PET/CT Parameters on Treatment Response and Prognosis in Locally Advanced Rectal Cancer Treated with Neoadjuvant Chemoradiotherapy. J Gastrointest Canc 53, 341–347 (2022). https://doi.org/10.1007/s12029-021-00608-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12029-021-00608-y