Abstract
Background
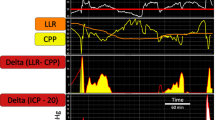
This prospective consecutive double-blinded randomized study investigated the effect of prostacyclin on pressure reactivity (PR) in severe traumatic brain injured patients. Other aims were to describe PR over time and its relation to outcome.
Methods
Blunt head trauma patients, Glasgow coma scale ≤8, age 15–70 years were included and randomized to prostacyclin treatment (n = 23) or placebo (n = 25). Outcome was assessed using the extended Glasgow outcome scale (GOSE) at 3 months. PR was calculated as the regression coefficient between the hourly mean values of ICP versus MAP. Pressure active/stable was defined as PR ≤0.
Results
Mean PR over 96 h (PRtot) was 0.077 ± 0.168, in the prostacyclin group 0.030 ± 0.153 and in the placebo group 0.120 ± 0.173 (p < 0.02). There was a larger portion of pressure-active/stable patients in the prostacyclin group than in the placebo group (p < 0.05). Intra-individual changes over time were common. PRtot correlated negatively with GOSE score (p < 0.04). PRtot was 0.117 ± 0.182 in the unfavorable (GOSE 1–4) and 0.029 ± 0.140 in the favorable outcome group (GOSE 5–8). Area under the curve for prediction of death (ROC) was 0.742 and for favorable outcome 0.628.
Conclusions
Prostacyclin influenced the PR in a direction of increased pressure stability and a lower PRtot was associated with improved outcome. The individual PR varied substantially over time. The predictive value of PRtot for outcome was not solid enough to be used in the clinical situation.




Similar content being viewed by others
References
Cold GE. Cerebral blood flow in acute head injury. The regulation of cerebral blood flow and metabolism during the acute phase of head injury, and its significance for therapy. Acta Neurochir Suppl (Wien). 1990;49:1–64.
Rosner MJ, Rosner SD, Johnson AH. Cerebral perfusion pressure: management protocol and clinical results. J Neurosurg. 1995;83:949–62.
Asgeirsson B, Grande PO, Nordstrom CH. A new therapy of post-trauma brain oedema based on haemodynamic principles for brain volume regulation. Intensive Care Med. 1994;20:260–7.
Grande PO. The “Lund Concept” for the treatment of severe head trauma–physiological principles and clinical application. Intensive Care Med. 2006;32:1475–84.
Rippe B, Haraldsson B. Transport of macromolecules across microvascular walls: the two-pore theory. Physiol Rev. 1994;74:163–219.
FitzGerald GA, Friedman LA, Miyamori I, O’Grady J, Lewis PJ. A double blind placebo controlled crossover study of prostacyclin in man. Life Sci. 1979;25:665–72.
Moncada S, Gryglewski R, Bunting S, Vane JR. An enzyme isolated from arteries transforms prostaglandin endoperoxides to an unstable substance that inhibits platelet aggregation. Nature. 1976;263:663–5.
Moncada S, Higgs EA, Vane JR. Human arterial and venous tissues generate prostacyclin (prostaglandin x), a potent inhibitor of platelet aggregation. Lancet. 1977;1:18–20.
Moncada S, Vane JR. The role of prostacyclin in vascular tissue. Fed Proc. 1979;38:66–71.
Moncada S, Vane JR. Arachidonic acid metabolites and the interactions between platelets and blood-vessel walls. N Engl J Med. 1979;300:1142–7.
Lundblad C, Grande PO, Bentzer P. Increased cortical cell loss and prolonged hemodynamic depression after traumatic brain injury in mice lacking the IP receptor for prostacyclin. J Cereb Blood Flow Metab. 2008;28:367–76.
Grande PO, Moller AD, Nordstrom CH, Ungerstedt U. Low-dose prostacyclin in treatment of severe brain trauma evaluated with microdialysis and jugular bulb oxygen measurements. Acta Anaesthesiol Scand. 2000;44:886–94.
Olivecrona M, Rodling-Wahlstrom M, Naredi S, Koskinen LO. Prostacyclin treatment in severe traumatic brain injury: a microdialysis and outcome study. J Neurotrauma. 2009;26:1251–62.
Aries MJ, Czosnyka M, Budohoski KP, et al. Continuous determination of optimal cerebral perfusion pressure in traumatic brain injury. Crit Care Med. 2012;40:2456–63.
Howells T, Elf K, Jones PA, et al. Pressure reactivity as a guide in the treatment of cerebral perfusion pressure in patients with brain trauma. J Neurosurg. 2005;102:311–7.
Steiner LA, Czosnyka M, Piechnik SK, et al. Continuous monitoring of cerebrovascular pressure reactivity allows determination of optimal cerebral perfusion pressure in patients with traumatic brain injury. Crit Care Med. 2002;30:733–8.
Lang EW, Chesnut RM. A bedside method for investigating the integrity and critical thresholds of cerebral pressure autoregulation in severe traumatic brain injury patients. Br J Neurosurg. 2000;14:117–26.
Czosnyka M, Pickard JD. Monitoring and interpretation of intracranial pressure. J Neurol Neurosurg Psychiatry. 2004;75:813–21.
Olivecrona M, Rodling-Wahlstrom M, Naredi S, Koskinen LO. Effective ICP reduction by decompressive craniectomy in patients with severe traumatic brain injury treated by an ICP-targeted therapy. J Neurotrauma. 2007;24:927–35.
Wilson JT, Pettigrew LE, Teasdale GM. Structured interviews for the Glasgow Outcome Scale and the extended Glasgow Outcome Scale: guidelines for their use. J Neurotrauma. 1998;15:573–85.
Moncada S, Vane JR. Prostacyclin and its clinical applications. Ann Clin Res. 1984;16:241–52.
Brandt L, Ljunggren B, Andersson KE, Hindfelt B, Uski T. Effects of indomethacin and prostacyclin on isolated human pial arteries contracted by CSF from patients with aneurysmal SAH. J Neurosurg. 1981;55:877–83.
Brandt L, Ljunggren B, Andersson KE, Hindfelt B, Uski T. Prostaglandin metabolism and prostacyclin in cerebral vasospasm. Gen Pharmacol. 1983;14:141–3.
Koskinen LO, Olivecrona M, Rodling-Wahlstrom M, Naredi S. Prostacyclin treatment normalises the MCA flow velocity in nimodipine-resistant cerebral vasospasm after aneurysmal subarachnoid haemorrhage: a pilot study. Acta Neurochir (Wien). 2009;151:595–9; discussion 599.
Wahlström M, Olivecrona M, Ahlm C, et al. Effects of prostacyclin on the early inflammatory response in patients with traumatic brain injury: a randomised clinical study. SpringerPlus. 2014;3:98.
Kurihara J, Sahara T, Kato H. Protective effect of beraprost sodium, a new chemically stable prostacyclin analogue, against the deterioration of baroreceptor reflex following transient global cerebral ischaemia in dogs. Br J Pharmacol. 1990;99:91–6.
de Souza M, Bouskela E. Arteriolar diameter and spontaneous vasomotion: importance of potassium channels and nitric oxide. Microvasc Res. 2013;90:121–7.
Rosenkranz AC, Rauch BH, Doller A, et al. Regulation of human vascular protease-activated receptor-3 through mRNA stabilization and the transcription factor nuclear factor of activated T cells (NFAT). Mol Pharmacol. 2011;80:337–44.
Pickard JD. Role of prostaglandins and arachidonic acid derivatives in the coupling of cerebral blood flow to cerebral metabolism. J Cereb Blood Flow Metab. 1981;1:361–84.
Sviri GE, Aaslid R, Douville CM, Moore A, Newell DW. Time course for autoregulation recovery following severe traumatic brain injury. J Neurosurg. 2009;111:695–700.
Czosnyka M, Smielewski P, Kirkpatrick P, Laing RJ, Menon D, Pickard JD. Continuous assessment of the cerebral vasomotor reactivity in head injury. Neurosurgery. 1997;41:11–7; discussion 17–9.
Jaeger M, Schuhmann MU, Soehle M, Meixensberger J. Continuous assessment of cerebrovascular autoregulation after traumatic brain injury using brain tissue oxygen pressure reactivity. Crit Care Med. 2006;34:1783–8.
Steiner LA, Pfister D, Strebel SP, Radolovich D, Smielewski P, Czosnyka M. Near-infrared spectroscopy can monitor dynamic cerebral autoregulation in adults. Neurocrit Care. 2009;10:122–8.
Zweifel C, Lavinio A, Steiner LA, et al. Continuous monitoring of cerebrovascular pressure reactivity in patients with head injury. Neurosurg Focus. 2008;25:E2.
Sanchez-Porras R, Santos E, Czosnyka M, Zheng Z, Unterberg AW, Sakowitz OW. ‘Long’ pressure reactivity index (L-PRx) as a measure of autoregulation correlates with outcome in traumatic brain injury patients. Acta Neurochir (Wien). 2012;154:1575–81.
Kirkness CJ, Mitchell PH, Burr RL, Newell DW. Cerebral autoregulation and outcome in acute brain injury. Biol Res Nurs. 2001;2:175–85.
Shahsavari S, McKelvey T, Ritzen CE, Rydenhag B. Cerebrovascular mechanical properties and slow waves of intracranial pressure in TBI patients. IEEE Trans Biomed Eng. 2011;58:2072–82.
Acknowledgments
The Department of Clinical Neuroscience University Hospital Research Found, Tore Nilsson Found, Kempe Found, Capio Research Found and Umeå University financially supported this study.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Koskinen, LO.D., Eklund, A., Sundström, N. et al. Prostacyclin Influences the Pressure Reactivity in Patients with Severe Traumatic Brain Injury Treated with an ICP-Targeted Therapy. Neurocrit Care 22, 26–33 (2015). https://doi.org/10.1007/s12028-014-0030-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-014-0030-8