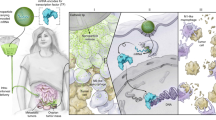
Abstract
M2-like tumor-associated macrophages (TAMs) play a significant role in immunosuppressive conditions in the tumor microenvironment (TME). TAM reprogramming, a dual-pronged therapy, reduces immunosuppression and induces immune favorable conditions in the TME. In this study, recombinant adenoviruses encoding active forms of interferon regulatory factor 3 (IRF3) were conjugated to zymosan particles to target phagocytic cells to create a pro-inflammatory immunomodulatory therapy. We determined TAM reprogramming by upregulation and downregulation of M1- and M2-associated genes, respectively, as well as cytokine and transcription factor expression in vitro. The overall shift to immune favorable conditions in the TME was suggested by metabolic, cytokine, and immune cell gene expression. Our data indicated that the zymosan:adenovirus (Zym:Ad) particle itself induced a shift from M2-like to M1-like TAMs, a shift in immune status of the TME, and systemic tumor immunity as determined using a double tumor melanoma mouse model and splenocyte functional assay. Notably, direct intratumoral injection of Zym:Ad IRF3 reduced tumor growth more significantly than Zym:Ad GFP, indicating additional therapeutic benefits due to incorporation of constant active IRF3.








Similar content being viewed by others
References
van der Woude LL, Gorris MA, Halilovic A, Figdor CG, de Vries IJM. Migrating into the tumor: a roadmap for T cells. Trends Cancer 2017;3(11):797–808.
Binnewies M, Roberts EW, Kersten K, Chan V, Fearon DF, Merad M, Coussens LM, Gabrilovich DI, Ostrand-Rosenberg S, Hedrick CC, et al. Understanding the tumor immune microenvironment (time) for effective therapy. Nat Med 2018;24(5):541–550.
Bonaventura P, Shekarian T, Alcazer V, Valladeau-Guilemond J, Valsesia-Wittmann S, Amigorena S, Caux C, Depil S. Cold tumors: a therapeutic challenge for immunotherapy. Front Immunol 2019;10:168.
Tähtinen S, Grönberg-Vähä-Koskela S, Lumen D, Merisalo-Soikkeli M, Siurala M, Airaksinen A, Vähä-Koskela M, Hemminki A. Combination immunotherapy with oncolytic adenovirus and adoptive T-cell transfer leads to systemic anti-tumor immunity and enhanced therapeutic efficacy in a preclinical melanoma model. Ann Oncol 2015;26:5.
Duan Q, Zhang H, Zheng J, Zhang L. Turning cold into hot: firing up the tumor microenvironment. Trends Cancer 2020;6(7):605–618.
Tang H, Qiao J, Fu Y-X. Immunotherapy and tumor microenvironment. Cancer Lett 2016; 370(1):85–90.
Gajewski TF, Corrales L, Williams J, Horton B, Sivan A, Spranger S. 2017. Cancer immunotherapy targets based on understanding the T cell-inflamed versus non-T cell-inflamed tumor microenvironment. Tumor immune microenvironment in cancer progression and cancer therapy, 19–31.
Chanmee T, Ontong P, Konno K, Itano N. Tumor-associated macrophages as major players in the tumor microenvironment. Cancers 2014;6(3):1670–1690.
Bardi GT, Smith MA, Hood JL. Melanoma exosomes promote mixed m1 and m2 macrophage polarization. Cytokine 2018;105:63–72.
Ceci C, Atzori MG, Lacal PM, Graziani G. Targeting tumor-associated macrophages to increase the efficacy of immune checkpoint inhibitors: a glimpse into novel therapeutic approaches for metastatic melanoma. Cancers 2020;12(11):3401.
Zhang Q-W, Liu L, Gong C-Y, Shi H-S, Zeng Y-H, Wang X-Z, Zhao Y-W, Wei Y-Q. Prognostic significance of tumor-associated macrophages in solid tumor: a meta-analysis of the literature. PloS ONE 2012;7(12):50946.
Macciò A, Gramignano G, Cherchi MC, Tanca L, Melis L, Madeddu C. Role of M1-polarized tumor-associated macrophages in the prognosis of advanced ovarian cancer patients. Sci Rep 2020;10(1): 1–8.
Pinto ML, Rios E, Durães C, Ribeiro R, Machado JC, Mantovani A, Barbosa MA, Carneiro F, Oliveira MJ. 2019. The two faces of tumor-associated macrophages and their clinical significance in colorectal cancer, Vol. 10.
Petrillo M, Zannoni GF, Martinelli E, Anchora Pedone L, Ferrandina G, Tropeano G, Fagotti A, Scambia G. Polarisation of tumor-associated macrophages toward M2 phenotype correlates with poor response to chemoradiation and reduced survival in patients with locally advanced cervical cancer. PLoS ONE 2015;10(9):0136654.
Conway EM, Pikor LA, Kung SH, Hamilton MJ, Lam S, Lam WL, Bennewith KL. Macrophages, inflammation, and lung cancer. Am J Respir Crit Care Med 2016;193(2):116–130.
Oshi M, Tokumaru Y, Asaoka M, Yan L, Satyananda V, Matsuyama R, Matsuhashi N, Futamura M, Ishikawa T, Yoshida K, et al. M1 macrophage and M1/M2 ratio defined by transcriptomic signatures resemble only part of their conventional clinical characteristics in breast cancer. Sci Rep 2020; 10(1):1–12.
De Palma M, Lewis CE. Macrophage regulation of tumor responses to anticancer therapies. Cancer Cell 2013;23(3):277–286.
Mitchem JB, Brennan DJ, Knolhoff BL, Belt BA, Zhu Y, Sanford DE, Belaygorod L, Carpenter D, Collins L, Piwnica-Worms D, et al. Targeting tumor-infiltrating macrophages decreases tumor-initiating cells, relieves immunosuppression, and improves chemotherapeutic responses. Cancer Res 2013;73(3):1128–1141.
Krausgruber T, Blazek K, Smallie T, Alzabin S, Lockstone H, Sahgal N, Hussell T, Feldmann M, Udalova IA. IRF5 promotes inflammatory macrophage polarization and TH1-TH17 responses. Nat Immunol 2011;12(3):231–238.
Musick M, Yu X. 2022. Manipulation of the tumor immuno-microenvironment via TAM-targeted expression of transcription factors. Immunol Res, 1–9.
Marabelle A, Tselikas L, De Baere T, Houot R. Intratumoral immunotherapy: using the tumor as the remedy. Ann Oncol 2017;28:33–43.
Larionova I, Kazakova E, Patysheva M, Kzhyshkowska J. Transcriptional, epigenetic and metabolic programming of tumor-associated macrophages. Cancers 2020;12(6):1411.
Cao X, Liang Y, Hu Z, Li H, Yang J, Hsu EJ, Zhu J, Zhou J, Fu Y-X. Next generation of tumor-activating type I IFN enhances anti-tumor immune responses to overcome therapy resistance. Nat Commun 2021;12(1):1–11.
Keshavarz A, Pourbagheri-Sigaroodi A, Zafari P, Bagheri N, Ghaffari SH, Bashash D. Toll-like receptors (TLRs) in cancer; with an extensive focus on TLR agonists and antagonists. IUBMB Life 2021;73(1):10–25.
Romieu-Mourez R, Solis M, Nardin A, Goubau D, Baron-Bodo V, Lin R, Massie B, Salcedo M, Hiscott J. Distinct roles for IFN regulatory factor (IRF)-3 and IRF-7 in the activation of antitumor properties of human macrophages. Cancer Res 2006;66(21):10576–10585.
Goubau D, Romieu-Mourez R, Solis M, Hernandez E, Mesplède T, Lin R, Leaman D, Hiscott J. Transcriptional re-programming of primary macrophages reveals distinct apoptotic and anti-tumoral functions of IRF-3 and IRF-7. Eur J Immunol 2009;39(2):527–540.
Maler MD, Nielsen PJ, Stichling N, Cohen I, Ruzsics Z, Wood C, Engelhard P, Suomalainen M, Gyory I, Huber M, et al. Key role of the scavenger receptor marco in mediating adenovirus infection and subsequent innate responses of macrophages. MBio 2017;8(4):00670–17.
Suzuki M, Rooney CM. Adenovirus immunity: X marks the spot. Mol Ther 2012;20(12): 2197–2198.
Brown GD, Taylor PR, Reid DM, Willment JA, Williams DL, Martinez-Pomares L, Wong SY, Gordon S. Dectin-1 is a major β-glucan receptor on macrophages. J Exp Med 2002;196(3):407–412.
de Graaff P, Berrevoets C, Röschm C, Schols HA, Verhoef K, Wichers HJ, Debets R, Govers C. Curdlan, zymosan and a yeast-derived β-glucan reshape tumor-associated macrophages into producers of inflammatory chemo-attractants. Cancer Immunol Immunother 2021;70(2):547–561.
Liu M, Luo F, Ding C, Albeituni S, Hu X, Ma Y, Cai Y, McNally L, Sanders MA, Jain D, et al. Dectin-1 activation by a natural product β-glucan converts immunosuppressive macrophages into an M1-like phenotype. J Immunol 2015;195(10):5055–5065.
Albeituni SH, Ding C, Liu M, Hu X, Luo F, Kloecker G, Bousamra M, Zhang H-G, Yan J. Yeast-derived particulate β-glucan treatment subverts the suppression of myeloid-derived suppressor cells (MDSC) by inducing polymorphonuclear MDSC apoptosis and monocytic MDSC differentiation to APC in cancer. J Immunol 2016;196(5):2167–2180.
Du Z, Kelly E, Mecklenbräuker I, Agle L, Herrero C, Paik P, Ivashkiv LB. Selective regulation of IL-10 signaling and function by zymosan. J Immunol 2006;176(8):4785–4792.
Maizel Jr JV, White DO, Scharff MD. The polypeptides of adenovirus: I evidence for multiple protein components in the virion and a comparison of types 2, 7A, and 12. Virology 1968;36(1):115–125.
Duits DE, Wellenstein MD, de Visser KE, Vol. 632. In vitro assessment of cancer cell-induced polarization of macrophages. New York: Elsevier; 2020, pp. 133–154.
Yuan R, Li S, Geng H, Wang X, Guan Q, Li X, Ren C, Yuan X. Reversing the polarization of tumor-associated macrophages inhibits tumor metastasis. Int Immunopharmacol 2017;49: 30–37.
Aguilera TA, Rafat M, Castellini L, Shehade H, Kariolis MS, Hui AB-Y, Stehr H, Von Eyben R, Jiang D, Ellies LG, et al. Reprogramming the immunological microenvironment through radiation and targeting AxL. Nat Commun 2016;7(1):1–14.
Lim JF, Berger H, Su I-H. Isolation and activation of murine lymphocytes. JoVE (Journal of Visualized Experiments) 2016;116:54596.
Bryant J, Day R, Whiteside TL, Herberman RB. Calculation of lytic units for the expression of cell-mediated cytotoxicity. J Immunol Methods 1992;146(1):91–103.
Osborn L, Kunkel S, Nabel GJ. Tumor necrosis factor alpha and interleukin 1 stimulate the human immunodeficiency virus enhancer by activation of the nuclear factor kappa B. Proc Natl Acad Sci 1989; 86(7):2336–2340.
Hiscott J. Triggering the innate antiviral response through IRF-3 activation. J Biol Chem 2007; 282(21):15325–15329.
Mancino A, Lawrence T. Nuclear factor-κ b and tumor-associated macrophages. Clin Cancer Res 2010;16(3):784–789.
Binnemars-Postma K, Bansal R, Storm G, Prakash J. Targeting the stat6 pathway in tumor-associated macrophages reduces tumor growth and metastatic niche formation in breast cancer. FASEB J 2018;32 (2):969–978.
Luby A, Alves-Guerra M-C. Targeting metabolism to control immune responses in cancer and improve checkpoint blockade immunotherapy. Cancers 2021;13(23):5912.
Ananieva E. Targeting amino acid metabolism in cancer growth and anti-tumor immune response. World J Biol Chem 2015;6(4):281.
Palmieri EM, Gonzalez-Cotto M, Baseler WA, Davies LC, Ghesquière B, Maio N, Rice CM, Rouault TA, Cassel T, Higashi RM, et al. Nitric oxide orchestrates metabolic rewiring in m1 macrophages by targeting aconitase 2 and pyruvate dehydrogenase. Nat Comm 2020;11(1):1–17.
Han S, Wang W, Wang S, Yang T, Zhang G, Wang D, Ju R, Lu Y, Wang H, Wang L. Tumor microenvironment remodeling and tumor therapy based on M2-like tumor associated macrophage-targeting nano-complexes. Theranostics 2021;11(6):2892.
Zhuang H, Dai X, Zhang X, Mao Z, Huang H. Sophoridine suppresses macrophage-mediated immunosuppression through TLR4/IRF3 pathway and subsequently upregulates CD8+ T cytotoxic function against gastric cancer. Biomed Pharmacother 2020;121:109636.
Hassan F, Lossie SL, Kasik EP, Channon AM, Ni S, Kennedy MA. A mouse model study of toxicity and biodistribution of a replication defective adenovirus serotype 5 virus with its genome engineered to contain a decoy hyper binding site to sequester and suppress oncogenic HMGA1 as a new cancer treatment therapy. PLoS ONE 2018;13(2):0192882.
Chan GC-F, Chan WK, Sze DM-Y. The effects of β-glucan on human immune and cancer cells. J Hematol Oncol 2009;2(1):1–11.
Mazzone M, Menga A, Castegna A. Metabolism and TAM functions—it takes two to tango. FEBS J 2018;285(4):700– 716.
Kumar V, Gabrilovich DI. Hypoxia-inducible factors in regulation of immune responses in tumour microenvironment. Immunology 2014;143(4):512–519.
You L, Wu W, Wang X, Fang L, Adam V, Nepovimova E, Wu Q, Kuca K. The role of hypoxia-inducible factor 1 in tumor immune evasion. Med Res Rev 2021;41(3):1622–1643.
Duguay D, Mercier F, Stagg J, Martineau D, Bramson J, Servant M, Lin R, Galipeau J, Hiscott J. In vivo interferon regulatory factor 3 tumor suppressor activity in B16 melanoma tumors. Cancer Res 2002;62(18):5148–5152.
Marabelle A, Andtbacka R, Harrington K, Melero I, Leidner R, de Baere T, Robert C, Ascierto PA, Baurain J-F, Imperiale M, et al. Starting the fight in the tumor: expert recommendations for the development of human intratumoral immunotherapy (HIT-IT). Ann Oncol 2018;29(11):2163–2174.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Author contribution
All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Musick, M., Yu, X. Immunotherapeutic effects of intratumorally injected Zymosan-Adenovirus conjugates encoding constant active IRF3 in a melanoma mouse model. Immunol Res 71, 197–212 (2023). https://doi.org/10.1007/s12026-022-09336-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12026-022-09336-2