Abstract
We report on a novel application of real-time reverse transcription-loop-mediated isothermal amplification (real-time RT-LAMP) to identify the presence of a specific body fluid using blood as a proof-of-concept model. By comparison with recently developed methods of body fluid identification, the RT-LAMP assay is rapid and requires only one simple heating-block maintained at a single temperature, circumventing the need for dedicated equipment. RNA was extracted from different body fluids (blood, semen, saliva, menstrual blood, sweat, and urine) for use in real-time RT-LAMP reaction. The 18S rRNA locus was used as the internal control and hemoglobin beta (HBB) as the blood-specific marker. Reverse transcription and LAMP reaction were performed in the same tube using a turbidimeter for real-time monitoring the reaction products within a threshold of 60 min. HBB LAMP products were only detected in blood and not in any of the other body fluid, but products from the 18S rRNA gene were detected in all the tested body fluids as expected. The limit of detection was a minimum of 10−5 ng total RNA for detection of both 18S rRNA and HBB. Augmenting the detection of RT-LAMP products was performed by separation of the products using gel electrophoresis and collecting the fluorescence of calcein. The data collected indicated complete concordance with the body fluid tested regardless of the method of detection used. This is the first application of real-time RT-LAMP to detect body fluid specific RNA and indicates the use of this method in forensic biology.
Similar content being viewed by others
Introduction
Body fluids are encountered frequently during a forensic investigation where it is standard practice to perform STR typing for human identification. The identification of the body fluid(s) from which the DNA profile arose is now possible using the mRNA from the sample [1, 2]. Analysis of RNA has been found to be a specific test for a range of body fluids [3] and both sensitive and stable [4–6]. Recent studies also have shown the potential of microRNA (miRNA) [7] and DNA methylation to identify body fluids through differential patterns [8].
Typically the RNA is reverse-transcribed into cDNA prior to PCR amplification. The presence or absence of tissue-specific markers is either monitored by the use of real-time PCR [9, 10] or the products detected by capillary electrophoresis [11]. These methods have shown real potential in forensic practice but are time consuming and require multiple steps with potential loss of sample. Furthermore, there is the inherent possibility of degradation of mRNA. A method yet to be applied to body fluid identification is reverse transcription-loop mediated isothermal amplification (RT-LAMP) where reverse transcription and subsequent LAMP reaction can be performed in one tube [12]. This rapid real-time detection by using RT-LAMP has been applied to the diagnosis of human viral infections [13, 14]. The combination of reverse transcription with the LAMP has a real potential application for the forensic detection of body fluids.
The LAMP technique was developed by Notomi et al. [12, 15] and has been used widely for rapid detection and identification of parasites and diseases [16–18] and even in determining the sex of embryonic animals [19]. The only two applications of LAMP in a forensic context to-date were detecting bacterial strains relevant to those in saliva [20] and identification of human DNA by LAMP combined with a colorimetric gold nanoparticle hybridization probe [21]. LAMP relies on DNA amplification via auto-cycling mediated by a DNA polymerase that displaces target strand DNA and creates new targets as it amplifies. The process requires two primer sets, inner and outer, which recognize six specific sites and thus provide more specificity than the traditional PCR (an animation of the process can be found at http://loopamp.eiken.co.jp/e/lamp/anim.html). The LAMP product is a combination of various lengths of amplified DNA (the description given is that of a cauliflower-like DNA structure) [12, 15]. While the reaction might appear complex, LAMP products can be generated within 1 h and at one single temperature, thus requiring no tube changes and one simple piece of equipment. The LAMP products are detected simply by conjugating the pyrophosphate produced with magnesium, which forms a white precipitate of magnesium pyrophosphate [22], the accumulation of which can be observed by the naked eye or detected by a turbidimeter in real-time [23]. As an alternative, the fluorescent dye calcein can also be used to detect the LAMP products [24, 25] as calcein combines with the manganese ions before the reaction. Manganese ions will conjugate with the pyrophosphate ions as they are produced during the LAMP reaction rather than calcein, and as free calcein combines with residual magnesium ions, a change in fluorescence can be monitored.
Harnessing the advantages of real-time RT-LAMP allowed the rapid determination of the body fluid type present in a sample using blood identification as a model system. The HBB gene was selected as the specific marker for blood [26], and a housekeeping gene (18S rRNA) as the control for the presence of total RNA [11]. The optimal primer sets of RT-LAMP for blood identification were designed and the different detection methods were tested. We hypothesized that this method may have superior sensitivity than current methods and be highly specific for a target locus. The potential added advantages of using one piece of equipment and speed of reaction were also reasons to examine this method in addition to applying the test to case samples.
Materials and methods
Sample collection
The body fluids used in this study included venous blood, saliva, semen, menstrual blood, sweat, and urine. These were collected from six volunteers between 20 and 40 years old for each body fluid using procedures approved by Institutional Review Board (IRB) of Central Police University in Taiwan. Volunteers were asked to rinse their mouth with distilled water before collecting the saliva samples. Menstrual blood was collected from a tampon from which the blood was squeezed into a collection tube. Sweat samples were collected into a sterile tube from the volunteers after they had exercised for 30 min. Animal blood samples from eight species (pig, dog, chicken, cow, goose, goat, cat, and rabbit), to detect any cross-species reaction, were kindly provided by the Livestock Research Institute, Council of Agriculture, in Taiwan. The collection of samples from six species (pig, chicken, cow, goose, goat and rabbit) was reviewed and approved by the Institutional Animal Care and Use Committee (IACUC); and samples of the other two species (dog and cat) were collected from the animal hospital with appropriate informed consent. All the liquid body fluids were stored at −20 °C immediately after collection.
Total RNA extraction and quantification
Total RNA was extracted from 50 μL of each liquid sample and eluted with 30 μL RNase-free water for each body fluid using the RNeasy® Mini Kit (Qiagen® Ltd., CA, USA) following the manufacturer’s protocol. RNase-free DNase (Qiagen®, Hilden, Germany) was added during the extraction to prevent the contamination of DNA. RNA was then quantified using the Nanodrop spectrophotometer (NanoDrop® ND-1000 UV spectrophotometer, Thermo Scientific, Wilmington, USA).
Marker selection and primer design for LAMP
The HBB gene was selected as the specific marker for blood and a housekeeping gene (18S rRNA) as the control. DNA sequences of 18S rRNA and HBB were extracted from GenBank (accession nos. U13369.1 and NM000518.4 respectively). The primers used in LAMP were designed using the LAMP primer designing software PRIMER EXPLORER V3 (http://primerexplorer.jp/elamp3.0.0/index.html). Based on recommendation for LAMP: the Tm ranged from 59 to 66 °C; to assist with stability the ends of the primers were designed to meet the requirement of free energy (∆G) less than −4 kcal/mol; GC content of primers was between 40 and 65 %; the distance ranged from 120 to 160 bases between the inner primer set; and the distance of the inner and outer primers ranged from 0 to 60 bases. Additionally, at least one primer was designed to span intron/exon junctions to prevent the amplifications from any contaminating DNA. A series of primer sequences were obtained and the optimal primer sets were selected from them after trials.
RT-LAMP
Reverse transcription and LAMP reactions were performed simultaneously in the same tube by using a Loopamp RNA Amplification Kit (Eiken Chemical Co. Ltd., Tochigi, Japan) and a Realtime turbidimeter LA-500 (Eiken Chemical Co. Ltd., Tochigi, Japan) following the manufacturer’s protocols. The total volume for a reaction was 25 μL consisting of: 40 μM for each of the inner primers named FIP and BIP, 5 μM for each of the outer primers named F3 and B3 [12], 12.5 μL reaction mix which including 40 mM Tris–HCl (pH 8.8), 20 mM KCl, 16 mM MgSO4, 20 mM (NH4)2SO4, 0.2 % Tween20, 1.6 M Betaine and 2.8 mM for each dNTPs, 1 U enzyme (a mixture of Bst DNA Polymerase and AMV reverse transcriptase) and approximately 20 ng of the total RNA.
The thermal program for the Realtime turbidimeter LA-500 was 65 °C for 60 min and then 80 °C for 5 min following the suggestions of the manufacturer. If an alternative method (electrophoresis or fluorescence) was used for detecting the products, the LAMP reaction was performed using a GeneAmp® PCR System 9700 thermal cycler (Life Technologies, NY, USA) with the same conditions as the Realtime turbidimeter.
RT-LAMP product detection
Real-time detection was performed using a Realtime turbidimeter LA-500 to measure the turbidity of the samples during the reaction. The threshold time (T t, min) is recorded as the time when the measurement calculated by moving average differentiation of the turbidity exceeds the threshold (0.1) according to manufacturer’s instructions.
Detection by gel electrophoresis was performed using an aliquot (3 µL) of the LAMP products separated on a 2 % agarose gel in 1X TBE buffer at 120 V and 400 mA for 40 min and then stained with the SYBR® Green I (Invitrogen™, Paisley, UK). The gel image was then photographed under UV transillumination (Syngene® GBox, MD, USA) using Genesnap® from Syngene® image acquisition software (Vision-Capt version 14.2).
Fluorescence detection was performed using 1 μL calcein (Eiken Chemical Co., Ltd., Tochigi, Japan) added to the RT-LAMP preparations following the recommendation of the manufacturer. The working concentration of calcein in the LAMP reaction is approximately 2 mM. Fluorescence could be observed by the naked eye using a handheld-UV lamp (wavelength 365 nm).
Applications on the non-probative forensic samples
To evaluate the potential of real-time RT-LAMP on forensic blood identification, 21 non-probative forensic samples were collected. These samples were screened by the presumptive blood tests, Kastle–Meyer test [27] and HemDirect Hemoglobin test (SERATEC®, Göttingen, Germany) [28], following standard methods. Total RNA ranging from 9 to 1242 ng in 30 μL was extracted from the samples of approximately 1 cm2 materials (swab, fabric or gauze) from a blood stained area, and 5 μL of each was used for blood identification by real-time RT-LAMP following the conditions provided above. These real casework traces are explained in the supplementary table (Online Resource 1).
Results
Primer design
During the preliminary stages a series of primer sets designed by PRIMER EXPLORER V3 were evaluated for their amplification efficiency and specificity. The optimal primer sets for 18S rRNA and HBB amplification were selected based on extensive trials. Sequences (5′–3′) of the primers used in the LAMP reaction are: ATTGACGGAAGGGCACCA (F3), TGCCAGAGTCTCGTTCGTTA (B3), CAATCCTGTCCGTGTCCGGGAGCCTGCGGCTTAATTTGAC (FIP) and AGCTCTTTCTCGATTCCGTGGGAGACAAATCGCTCCACCAAC (BIP) for 18S rRNA amplification; GCTGCACTGTGACAAGCT (F3), TGGACAGCAAGAAAGCGAG (B3), GCCAAAGTGATGGGCCAGCACCTGAGAACTTCAGGCTCCT (FIP) and AAGAATTCACCCCACCAGTGCAAGGGCATTAGCCACACCA (BIP) for HBB amplification. The primers named FIP (composed of F1c and F2) and BIP (composed of B1c and B2) were the inner primer set; F3 and B3 were the outer primer set [12]. The respective sites for these primers are shown in Fig. 1. The predicted size from F3 to B3c site for 18S rRNA and HBB were 197 and 192 bp, respectively.
Reproducibility and specificity of the assay
Subsequent to determining the optimal amplification conditions, the reproducibility and specificity of real-time RT-LAMP was analyzed in triplicate for both loci using one sample of each body fluid. The average and standard deviation of T t (min) are shown in Table 1. The RT-LAMP products from the 18S rRNA locus were detected in all the tested body fluids, as would be expected. The T t ranged from 36.0 ± 0.9 to 42.8 ± 1.3 min. HBB LAMP products were only detected in venous and menstrual blood within 33.1 ± 4.8 and 40.6 ± 3.3 min respectively; this is as would be expected if the method was specific for blood. No amplification products were observed for the negative control (NC). The results indicate that the repeat tests were reproducible in the triplicate assay, and specificity as expected was observed.
Additionally, the expressions of these two markers were analyzed for all the different body fluids collected from six individuals for each of the body fluids. A positive result (within 60 min) was observed for all samples from the 18S rRNA locus (Fig. 2), and the T t value (min) ranged from 40.9 ± 5.0 (for menstrual blood) to 51.1 ± 5.7 (for sweat). However, HBB expression was only detected for the venous (36.8 ± 4.1 min) and menstrual blood (46.6 ± 5.9 min), and the urine samples from two females (50.6 and 53.3 min). It was confirmed subsequently that these urine samples were collected during menstruation and therefore most likely mixed with their menstrual blood. This result further illustrates the reproducibility and specificity of these markers though individual variation was observed.
To detect any cross-reaction with other animal species, total RNA was extracted from the blood of the following eight species: pig, dog, chicken, cow, goose, goat, cat, and rabbit (triplicate samples were collected for each of the species). No HBB LAMP products were detected within 60 min of the reaction (this was the reaction time for the study), however a reaction product from one of the pig blood samples (not for the other two) was detected after 70.5 min.
Sensitivity analysis
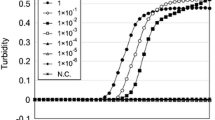
To determine the limit of detection for the test, total RNA from venous blood was serially diluted in triplicate using a tenfold dilution series down to a final dilution of 1 to 10−7 ng RNA. Products for both the 18S rRNA and HBB loci were observed from a minimum of 10−5 ng total RNA by real-time RT-LAMP within 60 min (Online Resource 2), and the T t values were 57.3 ± 3.5, and 56.5 ± 1 min respectively. These data illustrate the relatively high sensitivity of this assay.
Additionally to determine the limit of detection within a mixture of body fluids, total RNA from venous blood was mixed with RNA extracted from the other body fluids (semen, saliva, sweat, and urine) at different ratios of 9:1, 1:1, and 1:9. The amount of total RNA for each mixture was approximately 20 ng. The expression of both 18S rRNA and HBB was observed for all mixture preparations (Fig. 3) with the similar amplification pattern for each gene. These results indicated that there was no significant influence on the detection of blood HBB within a mixed sample.
Detection of RT-LAMP products by electrophoresis and fluorescence
To examine the alternative means of detecting RT-LAMP products, separation of products on an agarose gel and monitoring the presence of fluorescence for calcein were used. The ladder-like structure on agarose gel, and calcein fluorescence, was observed for 18S rRNA in all tested body fluids (Fig. 4). HBB products were only recorded with the venous and menstrual blood. These results were found to be concordant with the detection of the RT-LAMP products using a turbidimeter (real-time RT-LAMP). Monitoring the presence of fluorescence could be performed remote from the laboratory, such as at a crime scene as well as being performed in a laboratory environment.
Detection of LAMP products for 18S rRNA (a) and HBB (b) by agarose gel electrophoresis and calcein fluorescence. M DNA marker, VB venous blood, SE semen, SA saliva, MB menstrual blood, SW sweat, U urine, NC negative control. Conditions of the photography for the calcein fluorescence are ISO100, F/8 and 1/8 s for exposure
Applications for the identification of the non-probative forensic samples
The real-time RT-LAMP system was applied to the identification of body fluids from 21 non-probative forensic samples where it was suspected that the blood was present. The real casework traces are described in the supplementary material (Online Resource 1). The time intervals between sampling at the crime scenes and analyzing in this study ranged from about 1 month to 12 years. The original blood samples were observed on the surface of the substrates such as a mobile phone, bed, knife, and trouser pocket etc. All samples except sample 6 tested positive for the presence of blood using KM and/or HemDirect. For all these samples, expression of the 18S rRNA and HBB was detected within 60 min (Table 2); thus confirming the presence of blood. For sample 4, 5 and 6, results of the presumptive tests (KM or HemDirect tests) were either not concordant or unclear, however the presence of blood was confirmed using the real-time RT-LAMP assay. An example is sample 6, which was collected from a meat cleaver that was allegedly used as a weapon in an assault; it was suspected that the cleaver was subsequently washed. Forensic STR profiling from the blade of the cleaver matched the suspect using 16 loci (15 STR and the amelogenin) lending support (as did other relevant information) that the DNA profile was from an area of the cleaver that had been bloodstained, but the presence of blood was removed by washing. Our preliminary data indicate that the real-time RT-LAMP exhibits greater sensitivity than the KM and HemDirect tests for blood identification.
Discussion
Several studies have been performed dealing with mRNA or miRNA assays for body fluid identification. Typically the tissue-specific marker is either detected by real-time PCR or by capillary electrophoresis. These methods have shown an increase in the sensitivity of the test compared to previous methods and a real potential in forensic practice. By comparisons with the RT-LAMP used in this study however, these methods are time consuming and require multiple steps with potential loss of sample. Furthermore, the relative success of a multiplex mRNA-profiling system for the forensic identification of body fluids can often be unbalanced due to unequal expression of the different RNAs in a tissue [11]; though in general miRNAs are extremely conservative across species, however, some human miRNA are not human-specific [29]. It is also observed that the body fluid-specificities of some miRNAs identified, exhibit reported inconsistencies [7]. This was the rationale to explore an alternative method for body fluid typing.
This is the first application of real-time RT-LAMP to detect body fluid specific RNA and indicates the use of this method in forensic biology. In this study, identification of the blood was used as a proof-of-concept model, however, the expected limitations for expanding RT-LAMP technique to fluids other than blood will be the insufficient RNA quantity and quality from the trace or degraded samples. Therefore, the more sensitive conditions and strategies of RT-LAMP will be developed to overcome the obstacles.
Conclusions
We report on a novel application of real-time RT-LAMP to determine the presence of a body fluid, using blood as a model. The results showed that the method was reproducible, specific, sensitive and time-saving. An advantage to RT-LAMP is that the test could be performed using one piece of laboratory equipment, rather than a dedicated machine. It was also shown that detecting fluorescence due to the presence of calcein from the RT-LAMP products could be performed simply and is applicable to testing remotely from the laboratory such as at a crime scene. The assay was applied successfully to the putative identification of blood from 21 non-probative forensic samples. This is the first application of real-time RT-LAMP for body fluid detection and applied to a forensic science context. Based on the data using blood as a model system, there is real potential to apply this method to the identification of other body fluids.
Key points
-
1.
A novel method for the detection of blood as a model for body fluid identification is described using real-time RT-LAMP.
-
2.
Real-time RT-LAMP was found to be highly specific for blood.
-
3.
The limit of detection was in the range of 10−5 ng of RNA.
-
4.
The method was tested successfully on case samples and proved more sensitive than standard presumptive tests for blood.
References
Virkler K, Lednev IK. Analysis of body fluids for forensic purposes: from laboratory testing to non-destructive rapid confirmatory identification at a crime scene. Forensic Sci Int. 2009;188(1–3):1–17.
Bauer M. RNA in forensic science. Forensic Sci Int Genet. 2007;1(1):69–74.
Roeder AD, Haas C. mRNA profiling using a minimum of five mRNA markers per body fluid and a novel scoring method for body fluid identification. Int J Leg Med. 2013;127(4):707–21.
Zubakov D, Hanekamp E, Kokshoorn M, Van Ijcken W, Kayser M. Stable RNA markers for identification of blood and saliva stains revealed from whole genome expression analysis of time-wise degraded samples. Int J Leg Med. 2008;122(2):135–42.
Zubakov D, Kokshoorn M, Kloosterman A, Kayser M. New markers for old stains: stable mRNA markers for blood and saliva identification from up to 16-year-old stains. Int J Leg Med. 2009;123(1):71–4.
Bauer M, Polzin S, Patzelt D. Quantification of RNA degradation by semi-quantitative duplex and competitive RT-PCR: a possible indicator of the age of bloodstains? Forensic Sci Int. 2003;138(1–3):94–103.
Wang Z, Luo H, Pan X, Liao M, Hou Y. A model for data analysis of microRNA expression in forensic body fluid identification. Forensic Sci Int Genet. 2012;6(3):419–23.
Vidaki A, Daniel B, Court DS. Forensic DNA methylation profiling—potential opportunities and challenges. Forensic Sci Int Genet. 2013;7(5):499–507.
Bauer M, Patzelt D. Identification of menstrual blood by real time RT-PCR: technical improvements and the practical value of negative test results. Forensic Sci Int. 2008;174(1):55–9.
Haas C, Klesser B, Maake C, Bar W, Kratzer A. mRNA profiling for body fluid identification by reverse transcription endpoint PCR and realtime PCR. Forensic Sci Int Genet. 2009;3(2):80–8.
Lindenbergh A, de Pagter M, Ramdayal G, Visser M, Zubakov D, Kayser M, et al. A multiplex (m) RNA-profiling system for the forensic identification of body fluids and contact traces. Forensic Sci Int Genet. 2012;6(5):565–77.
Notomi T, Okayama H, Masubuchi H, Yonekawa T, Watanabe K, Amino N, et al. Loop-mediated isothermal amplification of DNA. Nucleic Acids Res. 2000;28(12):e63.
Inoue S, Muigai AW, Kubo T, Sang R, Morita K, Mwau M. A real-time reverse transcription loop-mediated isothermal amplification assay for the rapid detection of yellow fever virus. J Virol Methods. 2013;193(1):23–7.
Wei H, Zeng J, Deng C, Zheng C, Zhang X, Ma D, et al. A novel method of real-time reverse-transcription loop-mediated isothermal amplification developed for rapid and quantitative detection of human astrovirus. J Virol Methods. 2013;188(1–2):126–31.
Nagamine K, Hase T, Notomi T. Accelerated reaction by loop-mediated isothermal amplification using loop primers. Mol Cell Probes. 2002;16(3):223–9.
Mahony J, Chong S, Bulir D, Ruyter A, Mwawasi K, Waltho D. Development of a sensitive loop-mediated isothermal amplification assay that provides specimen-to-result diagnosis of respiratory syncytial virus infection in 30 minutes. J Clin Microbiol. 2013;51(8):2696–701.
Xu L, Kong J. A multiplexed nucleic acid microsystem for point-of-care detection of HIV co-infection with MTB and PCP. Talanta. 2013;117:532–5.
Yamazaki W, Mioulet V, Murray L, Madi M, Haga T, Misawa N, et al. Development and evaluation of multiplex RT-LAMP assays for rapid and sensitive detection of foot-and-mouth disease virus. J Virol Methods. 2013;192(1–2):18–24.
Zoheir K, Allam A. A rapid improved method for sexing embryo of water buffalo. Theriogenology. 2011;76(1):83–7.
Nakanishi H, Ohmori T, Hara M, Takada A, Shojo H, Adachi N, et al. A simple identification method of saliva by detecting streptococcus salivarius using loop-mediated isothermal amplification. J Forensic Sci. 2011;56(s1):S158–61.
Watthanapanpituck K, Kiatpathomchai W, Chu E, Panvisavas N. Identification of human DNA in forensic evidence by loop-mediated isothermal amplification combined with a colorimetric gold nanoparticle hybridization probe. Int J Leg Med. 2014;128(9):923–31.
Mori Y, Nagamine K, Tomita N, Notomi T. Detection of loop-mediated isothermal amplification reaction by turbidity derived from magnesium pyrophosphate formation. Biochem Biophys Res Commun. 2001;289(1):150–4.
Mori Y, Kitao M, Tomita N, Notomi T. Real-time turbidimetry of LAMP reaction for quantifying template DNA. J Biochem Biophys Methods. 2004;59(2):145–57.
Tomita N, Mori Y, Kanda H, Notomi T. Loop-mediated isothermal amplification (LAMP) of gene sequences and simple visual detection of products. Nat Protoc. 2008;3(5):877–82.
Breuer W, Epsztejn S, Millgram P, Cabantchik IZ. Transport of iron and other transition metals into cells as revealed by a fluorescent probe. Am J Physiol. 1995;268(6):C1354–61.
Levings PP, Bungert J. The human β-globin locus control region. Eur J Biochem. 2002;269(6):1589–99.
Gaensslen RE. Sourcebook in forensic serology, immunology, and biochemistry. US Department of Justice, National Institute of Justice; 1983.
Misencik A, Laux DL. Validation study of the seratec hemdirect hemoglobin assay for the forensic identification of human blood. MAFS Newslett. 2007;36(2):18–26.
Zubakov D, Boersma AWM, Choi Y, van Kuijk PF, Wiemer EAC, Kayser M. MicroRNA markers for forensic body fluid identification obtained from microarray screening and quantitative RT-PCR confirmation. Int J Leg Med. 2010;124(3):217–26.
Acknowledgments
This study was supported by the Ministry of Science and Technology in Taiwan (NSC 101-2320-B-015-001 and NSC 102-2628-B-015-001-MY2). Animal blood samples were kindly provided by Livestock Research Institute, Council of Agriculture in Taiwan.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Su, CW., Li, CY., Lee, J.CI. et al. A novel application of real-time RT-LAMP for body fluid identification: using HBB detection as the model. Forensic Sci Med Pathol 11, 208–215 (2015). https://doi.org/10.1007/s12024-015-9668-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12024-015-9668-6