Abstract
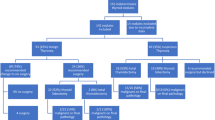
Molecular tests and mutational panels such as Afirma Gene Expression Classifier (GEC) and ThyroSeq, respectively, have been used to help risk stratify cytologically indeterminate thyroid nodules with the aim to reduce unnecessary surgeries. We studied the effect of molecular testing on the rate of surgical resection in these nodules. Thyroid nodules with indeterminate (Bethesda III/IV) cytology that underwent molecular testing (GEC or ThyroSeq) at our institution between June 2012 and August 2016 were retrospectively reviewed. We collected demographics, cytology diagnoses, molecular test results, and whether surgical resection was performed. Two hundred eighty-three nodules met inclusion criteria: 202 nodules tested with GEC and 81 tested with ThyroSeq. In the cohort of GEC-tested nodules, 99/202 (49%) yielded “suspicious” and 103/202 (51%) yielded “benign” results, with an overall resection rate of 70/99 (71%) in “suspicious” versus 13/103 (13%) in “benign” nodules. In the cohort of ThyroSeq-tested nodules, 13/81 (16%) of nodules yielded a “high-risk mutation” and 68/81 (84%) of nodules yielded “no high-risk mutation,” with overall resection rates of 11/13 (85%) and 30/68 (44%), respectively. Rates of resection were higher for Bethesda IV than for III nodules, regardless of molecular results. For both GEC and ThyroSeq, molecular test results seemed to correlate with the rate of resection at our institution. Rates of resection for cytologically indeterminate nodules that were “benign” or “no high-risk mutation” appeared to differ from those that were “suspicious” or “high-risk mutation” on molecular panel testing by GEC and ThyroSeq, respectively. Our findings support that molecular test results are impacting management.

Similar content being viewed by others
References
Cibas, E.S. & Ali, S.Z. The Bethesda System for Reporting Thyroid Cytopathology. Thyroid 19, 1159–1165 (2009).
Haugen, B.R., et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 26, 1-133 (2016).
Harrison, G., Sosa, J.A. & Jiang, X. Evaluation of the Afirma Gene Expression Classifier in Repeat Indeterminate Thyroid Nodules. Arch Pathol Lab Med 141, 985–989 (2017).
Roychoudhury, S., et al. How “suspicious” is that nodule? Review of “suspicious” Afirma gene expression classifier in high risk thyroid nodules. Diagn Cytopathol 45, 308–311 (2017).
Chaudhary, S., Hou, Y., Shen, R., Hooda, S. & Li, Z. Impact of the Afirma Gene Expression Classifier Result on the Surgical Management of Thyroid Nodules with Category III/IV Cytology and Its Correlation with Surgical Outcome. Acta Cytol 60, 205–210 (2016).
Livhits, M.J., et al. Gene Expression Classifier vs Targeted Next-Generation Sequencing in the Management of Indeterminate Thyroid Nodules. J Clin Endocrinol Metab 103, 2261–2268 (2018).
Valderrabano, P., et al. Evaluation of ThyroSeq v2 performance in thyroid nodules with indeterminate cytology. Endocr Relat Cancer 24, 127–136 (2017).
Taye, A., et al. Clinical performance of a next-generation sequencing assay (ThyroSeq v2) in the evaluation of indeterminate thyroid nodules. Surgery 163, 97–103 (2018).
Hang, J.F., Westra, W.H., Cooper, D.S. & Ali, S.Z. The impact of noninvasive follicular thyroid neoplasm with papillary-like nuclear features on the performance of the Afirma gene expression classifier. Cancer Cytopathol 125, 683–691 (2017).
Samulski, T.D., LiVolsi, V.A., Wong, L.Q. & Baloch, Z. Usage trends and performance characteristics of a “gene expression classifier” in the management of thyroid nodules: An institutional experience. Diagn Cytopathol 44, 867–873 (2016).
Wong, K.S., et al. Noninvasive Follicular Variant of Papillary Thyroid Carcinoma and the Afirma Gene-Expression Classifier. Thyroid 26, 911–915 (2016).
Valderrabano, P., et al. Evaluation of ThyroSeq v2 performance in thyroid nodules with indeterminate cytology. Endocrine-Related Cancer (2017).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jug, R., Parajuli, S., Ahmadi, S. et al. Negative Results on Thyroid Molecular Testing Decrease Rates of Surgery for Indeterminate Thyroid Nodules. Endocr Pathol 30, 134–137 (2019). https://doi.org/10.1007/s12022-019-9571-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12022-019-9571-x