Abstract
Purpose
The aim of this study was evaluate biochemical incomplete response (BIR) in Middle Eastern differentiated thyroid cancer (DTC), identify factors that could predict BIR before radioactive iodine (RAI) ablation and to investigate the long-term clinical outcome of DTC patient exhibiting BIR to initial therapy.
Methods
We retrospectively evaluated 1286 DTCs from Middle Eastern ethnicity who underwent total thyroidectomy and RAI therapy. Demograpic and clinico-pathological factors predicting BIR were evaluated. The outcome of these patients was analyzed using primary outcome of structural disease and disease-free survival (DFS).
Results
With a median follow-up of 10 years, 266 (20.7%) patients had BIR. High pre-ablation stimulated thyroglobulin (presTg), presence of lymph node metastasis, male gender and delayed initial RAI therapy (≥3 months) after thyroidectomy were significant independent predictors of BIR. Upon evaluating long-term clinical outcomes in 266 patients with BIR, we found 36.8% of patients developed structural disease. Male sex (OR = 1.56; 95% CI = 1.05–2.30; p = 0.0272) and increasing Tg after initial therapy (OR = 4.25; 95% CI = 1.93–10.82; p = 0.0001) were independent risk factors for structural disease in patients with BIR. DFS was significantly worse if both these risk factors existed concomitantly (p < 0.0001).
Conclusion
To achieve the fair efficacy of RAI therapy, early prediction of BIR before RAI ablation is desirable. Our finding of the clinico-pathological factors (high presTg level, LNM, delayed RAI therapy and male gender) could serve as easy and robust early predictors of BIR. In addition, DTC patients exhibiting BIR had a high risk of structural disease and hence personalized management approach would be preferable for BIR patients to ensure best clinical outcome.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Differentiated thyroid cancer (DTC), which includes papillary and follicular thyroid cancers, is the most common endocrine malignancy and accounts for 90% of all thyroid malignancies [1,2,3,4]. Therapy modalities of DTC mainly include surgery, radioactive iodine (RAI) therapy and thyrotropin (TSH) suppression therapy [5,6,7]. RAI therapy plays a crucial role in the purge of potential residual thyroid cancer and reducing risk of mortality [7, 8]. DTC, with appropriate therapy, tend to have excellent prognosis [7, 9, 10]. However, recurrence and persistence can occur several decades later [11,12,13]. Patients having persistent radioiodine avid lesions usually require long-term surveillance and repeated high dose of RAI [14, 15]. Therefore, it is very important to identify risk factors to predict disease persistence for DTC in order to help clinician to determine the most appropriate follow-up for these patients.
One of the risk assessment tools for persistence and recurrence is dynamic risk stratification (DRS) [7, 16, 17]. Based on an optimal response to initial therapy, DRS is composed of four response groups: excellent, indeterminate, biochemical incomplete and structural incomplete. Biochemical incomplete response (BIR) classification was defined as having persistently abnormal suppressed and/or stimulated thyroglobulin (Tg) or rising anti-Tg antibodies (TgAb) without structural evidence of disease [7]. Patients exhibiting BIR have shown to have a high risk of structural recurrent and persistent disease [18, 19].
Data about BIR in Middle Eastern DTC is limited. Therefore, we conducted this retrospective study on a large cohort of Middle Eastern DTC to determine: 1. The prevalence of BIR in this cohort, 2. Clinico-pathological parameters to predict BIR after surgery and before RAI, and 3. Long-term clinical outcomes of patient with BIR. Furthermore, we tried to investigate the relationship between the timing of initiating RAI therapy and BIR in this cohort.
Materials and methods
Clinical cohort
One-thousand eight-hundred and twenty-two DTC patients diagnosed between 1988 and 2018 at King Faisal Specialist Hospital and Research Center (Riyadh, Saudi Arabia) were available to be included in the study. The patients were included in the study after considering the following inclusion and exclusion criteria:
Inclusion criteria
-
1.
Pathologically proven DTC post total thyroidectomy
-
2.
Received radioactive iodine ablation post surgery
-
3.
Adequate clinical follow-up data for at least 12 months is available
Exclusion criteria
-
1.
Less than total thyroidectomy (n = 63)
-
2.
No radioactive iodine ablation received (n = 183)
-
3.
Patients with elevated TgAb during follow-up (n = 121)
-
4.
Patients with persistent positive WBS (n = 118)
-
5.
Patients with follow-up duration less than 12 months (n = 51)
After applying the inclusion and exclusion criteria, 1286 DTC patients were included for the final analysis. The Institutional Review Board of the hospital approved this study and since only retrospective patient data were used, the Research Advisory Council (RAC) provided waiver of consent under project RAC # 221 1168 and # 2110 031. The study was conducted in accordance with the Declaration of Helsinki.
Clinico-pathological and follow-up data
Baseline clinico-pathological data were collected from case records and have been summarized in Table 1. Staging of DTC was performed using the eighth edition of American Joint Committee on Cancer (AJCC) staging system [16]. Following initial surgery, all patients had pre-ablative stimulated Tg (presTg) evaluated before receiving RAI therapy. Patients were stratified into low, intermediate and high risk based on 2015 ATA guidelines [7]. Low-risk DTC patients were followed up annually, intermediate risk patients were followed up at 6 months’ intervals and high risk patients were followed up at 3 months’ intervals. At each follow-up, neck ultrasound, thyroid function tests, thyroglobulin (Tg) levels and thyroglobulin antibodies were performed. In addition, for high risk patients, whole body scan (WBS) and/or PET CT scan were performed to identify tumor persistence/recurrence. Patients with stimulated Tg of less than 10.0 ng/ml with no clinical or imaging evidence of tumors were considered complete treatment responses. BIR was considered in patients with a raised stimulated serum Tg level (>10 ng/ml) and a negative WBS.
BRAF and TERT mutation analysis
BRAF and TERT mutation data for the DTC cohort was available from our previous studies [20, 21].
Statistical analysis
The associations between clinico-pathological variables and BIR was performed using contingency table analysis and Chi square tests or Mann-Whitney U test for categorical and continuous variables, respectively. Disease-free survival (DFS) was determined using Kaplan-Meier estimates. DFS was defined as the time from diagnosis to the occurrence of recurrent disease or death. Logistic regression analysis was used for analyzing the prognostic factors that could predict BIR and structural persistent disease, in univariate and multivariate manner. Two-sided tests were used for statistical analyses with a limit of significance defined as p value < 0.05. Data analyses were performed using the JMP14.0 (SAS Institute, Inc., Cary, NC) software package.
Receiver operating characterisitcs (ROC) curve analysis was performed using MedCalc software, version 10.4.7.0 for Windows (MedCalc, Ostend, Belgium).
Results
Patient and tumor characteristics
Mean age of the entire cohort was 38.9 years, with a male: female ratio of 1:3. 32.7% (421/1286) of tumors were bilateral and 49.9% (642/1286) were multifocal. Extrathyroidal extension was noted in 42.9% (552/1286) of DTCs. Regional lymph node metastasis (LNM) was noted in 51.6% (664/1286) of cases and distant metastasis at diagnosis was present in 5.6% (72/1286). Frequency of BRAF and TERT mutations was 52.2% (672/1286) and 11.7% (150/1286), respectively. 49.0% (630/1286) of the DTC patients received RAI therapy within 3 months of surgery (Table 1).
Clinico-pathological characteristics associated with BIR
BIR was noted in 20.7% (266/1286) of DTCs and was significantly associated with male gender (p = 0.0105), larger tumor size (p = 0.0003), bilateral tumors (p = 0.0025), multifocality (p = 0.0214), extrathyroidal extension (p = 0.0013), lymph node metastasis (p < 0.0001), distant metastasis at diagnosis (p < 0.0001), advanced tumor stage (p = 0.0025), higher presTg levels (p < 0.0001), ATA high-risk category (p = 0.0089) and TERT mutation (p < 0.0001). Interestingly, we also found a significant association between BIR and ≥ three months interval to initiation of RAI therapy after surgery (p < 0.0001) (Table 2).
All the significant variables for BIR on univariate analysis were included for multivariate analysis, except for stage since it depends on other variables such as extrathyroidal extension, LNM and distant metastasis (which are already included for multivariate analysis). On multivariate logistic regression analysis, male gender (Odds ratio (OR) = 1.60; 95% confidence interval (CI) = 1.07–2.39; p = 0.0231), lymph node metastasis (LNM) (OR = 1.61; 95% CI = 1.06–2.45; p = 0.0267), higher presTg levels (OR = 1.05; 95% CI = 1.04–1.06; p < 0.0001) and interval to initiation of RAI therapy of ≥ three months (OR = 2.24; 95% CI = 1.51–3.32; p < 0.0001) were found to be independent predictors of BIR (Table 3).
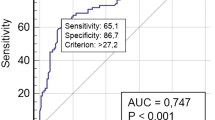
Diagnostic accuracy of presTg for predicting BIR
ROC curve was drawn to determine the diagnostic accuracy and cut-off value of presTg in identifying BIR in our cohort. Area under the curve (AUC) was 0.920 (95% CI = 0.904–0.934, p < 0.001) (Fig. 1). From the available cut-off values for presTg, the best cut-off value was ≥10 ng/ml (sensitivity = 95.1%, specificity = 81.3%). Negative predictive value (NPV) and positive predictive value (PPV) for BIR at presTg cut-off of 10 ng/ml were 98.5% and 57.0%, respectively. However, a cut-off of ≥ 6.9 ng/ml had a higher sensitivity (96.2%) and a reasonable specificity (73.8%), with NPV and PPV of 99.2% and 46.0%, respectively. The unadjusted odds ratio for BIR at presTg level of 10 ng/ml and 6.9 ng/ml was 84.5 (95% CI = 47.3–150.7, p < 0.0001) and 101.6 (95% CI = 44.7–230.7, p < 0.0001), respectively.
Long-term clinical outcomes in patients with BIR
We next sought to determine the incidence and associations of structural disease in patients with BIR during follow-up. During a median follow-up of 9.8 years, 36.8% (98/266) of patients with BIR developed structural disease. On multivariate logistic regression analysis, male gender (OR = 1.56; 95% CI = 1.05–2.30; p = 0.0272) and increasing Tg levels during follow-up (OR = 4.25; 95% CI = 1.93–10.82; p = 0.0001) were independent predictors of structural disease in patients with BIR (Table 4).
Based on the number of independent risk factors (male gender and increasing Tg levels during follow-up), patients were divided into 3 groups: low risk (no risk factors); intermediate risk (any one risk factors); and high risk (both risk factors). Risk stratification was performed for 266 patients with BIR with regards to DFS. 36.8% (98/266), 51.1% (136/266) and 12.0% (32/266) of patients were classified as low-, intermediate- and high-risk, respectively. 10-year DFS rates in the low-, intermediate- and high-risk groups were 82.3%, 50.6%, and 21.4%, respectively. DFS was significantly different among the three risk group, being worst in high-risk group (p < 0.0001) (Fig. 2).
Discussion
RAI therapy is a well-established therapeutic modality for DTC patients. Despite excellent prognosis, a significant percentage of DTCs develop biochemical incomplete response (BIR) [18, 22, 23]. Data on prevalence of BIR, clinico-pathological and biochemical risk factors to predict BIR after surgery and before radioiodine ablation in DTC patients from Middle Eastern ethnicity is not fully explored. Therefore, we conducted this retrospective study to evaluate BIR prevalence as well as predictive markers after surgery and before RAI therapy. In addition, we also evaluated long-term clinical outcomes of DTC patients showing BIR following initial therapy.
In this retrospective study of 1286 DTC patients, we noted that 20.7% (266/1286) of the patients show BIR. This incidence is consistent with a previous report [24]. However, a recent study has identified higher incidence (~36%) of BIR and have attributed this to the fact that most of their patients belong to intermediate or high risk categories [18]. In our study as well, ~90% of BIR patients are in the intermediate or high-risk category.
We further evaluated multiple clinico-pathological, biochemical and molecular markers to predict the risk of BIR before radioiodine ablation. In our study, we noted four risk factors as significant predictors of BIR on multivariant analysis: presTg, the time interval between thyroidectomy and first dose of RAI therapy (≥ 3 months), male gender, and LNM. Several previous reports have identified the impact of post-operative TSH stimulated Tg in predicting persistent and/or recurrent disease in DTC [25,26,27,28]. Our result is consistent with a recent study that found BIR risk to be very high in patients who had high presTg [18].
In our study, we found that presTg ≥6.9 ng/ml had 96.2% sensitivity to predict BIR prior to RAI ablation. We further noted that low presTg (<6.9 ng/ml) had excellent NPV (99.2%) to rule out BIR. We found reasonable specificity for presTg cutoff ≥10 ng/dl (specificity = 80.6%). As opposed to high NPV, the PPV of presTg over 10 ng/ml was quite low (57%). However, the main value of presTg is as a negative predictor of BIR when presTg values are low. Although the predictive value of presTg below 10 ng/ml (which is routinely used) has been demonstrated in our analysis, it is likely that lower cutoffs such as 6.9 ng/ml suggested by ROC curve would demonstrate an even higher NPV, albeit for a smaller group of patients.
Interestingly, our study has identified that the timing of initiating radioiodine adjuvant therapy could be a significant predictor of BIR in patients from this ethnicity. Although, the current DTC guidelines have no recommendation for timing of RAI [7], several previous studies have explored the relationship between RAI initiating time and DTC clinical outcome with conflicting conclusions [23, 29,30,31]. Our study showed that delayed initial RAI (≥ 3 months after thyroidectomy) was an independent predictor of BIR in this cohort.
Another interesting finding in this study was the association between BIR and male gender. Sex disparity in the incidence of DTC and its effect have been well documented [32,33,34]. However, the impact of gender on DTC from Middle Eastern ethnicity appears to be more pronounced as we have identified in our recent study [35] that male sex was an independent prognostic factor for recurrence-free survival in PTC.
We found that presence of LNM was an independent predictor of BIR in this cohort. This is in concordance with several previous studies, where LNM was shown to be associated with unfavorable outcome in DTC patients [36,37,38].
We further sought to evaluate the long-term outcome of BIR patients in this cohort. With a median follow-up of 9.8 years, 36% of patients had structural disease, which was found to be significantly associated with male gender and increasing Tg after initial therapy even in multivariant analysis. According to the number of risk factors, risk stratification related to poor DFS was attempted: low-risk group (having no risk factor), intermediate-risk-group (having any one risk factor), and high-risk group (having both risk factors). 10-year DFS rates in the low-, intermediate- and high-risk groups were 82.3%, 50.6%, and 21.4%, respectively. DFS was significantly different among the three risk groups, being worst in the high-risk group. Our findings suggest that a risk adaptive management in patients with BIR could be beneficial.
Our research has a few limitations. First, it was a single-center, retrospective study due to which bias cannot be excluded. Second, the study involved a specific ethnicity, which could prevent generalizing the findings on other patient populations.
Conclusion
We have identified easy and robust markers to predict BIR. We found that high presTg level, LNM, delayed RAI therapy (≥ 3 months) and male gender warrant higher future probabity of BIR even before RAI therapy. In addition, DTC patients exhibiting BIR had a high risk of structure disease and hence risk adaptive management is encouraged for BIR patients. Thorough disease surveillance is required for BIR patients and close attention should be given to male patients and patients with increasing Tg since they significantly impact clinical outcome in BIR patients.
References
J.A. Fagin, S.A. Wells Jr, Biologic and clinical perspectives on thyroid cancer. N. Engl. J. Med. 375, 1054–1067 (2016)
H. Lim, S.S. Devesa, J.A. Sosa, D. Check, C.M. Kitahara, Trends in thyroid cancer incidence and mortality in the United States, 1974–2013. JAMA 317, 1338–1348 (2017)
A. Miranda-Filho, J. Lortet-Tieulent, F. Bray, B. Cao, S. Franceschi, S. Vaccarella et al. Thyroid cancer incidence trends by histology in 25 countries: a population-based study. Lancet Diabetes Endocrinol. 9, 225–234 (2021)
L. Davies, H.G. Welch, Increasing incidence of thyroid cancer in the United States, 1973–2002. JAMA 295, 2164–2167 (2006)
F. Nabhan, P.H. Dedhia, M.D. Ringel, Thyroid cancer, recent advances in diagnosis and therapy. Int. J. Cancer 149, 984–992 (2021)
S. Filetti, C. Durante, D. Hartl, S. Leboulleux, L. Locati, K. Newbold et al. Thyroid cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 30, 1856–1883 (2019)
B.R. Haugen, E.K. Alexander, K.C. Bible, G.M. Doherty, S.J. Mandel, Y.E. Nikiforov et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid 26, 1–133 (2016)
A.M. Sawka, K. Thephamongkhol, M. Brouwers, L. Thabane, G. Browman, H.C. Gerstein, A systematic review and metaanalysis of the effectiveness of radioactive iodine remnant ablation for well-differentiated thyroid cancer. J. Clin. Endocrinol. Metab. 89, 3668–3676 (2004)
T. Carling, R. Udelsman, Thyroid cancer. Annu. Rev. Med. 65, 125–137 (2014)
W.R. Burns, M.A. Zeiger. Differentiated thyroid cancer. Semin Oncol: Elsevier; 2010. p. 557-566
C. Durante, T. Montesano, M. Torlontano, M. Attard, F. Monzani, S. Tumino et al. Papillary thyroid cancer: time course of recurrences during postsurgery surveillance. J. Clin. Endocrinol. Metab. 98, 636–642 (2013)
L. Lamartina, T. Montesano, F. Trulli, M. Attard, M. Torlontano, R. Bruno et al. Papillary thyroid carcinomas with biochemical incomplete or indeterminate responses to initial treatment: repeat stimulated thyroglobulin assay to identify disease-free patients. Endocrine 54, 467–475 (2016)
R.H. Grogan, S.P. Kaplan, H. Cao, R.E. Weiss, L.J. DeGroot, C.A. Simon et al. A study of recurrence and death from papillary thyroid cancer with 27 years of median follow-up. Surgery 154, 1436–1447 (2013)
D.S. Cooper, G.M. Doherty, B.R. Haugen, R.T. Kloos, S.L. Lee, S.J. Mandel et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association (ATA) guidelines taskforce on thyroid nodules and differentiated thyroid cancer. Thyroid 19, 1167–1214 (2009)
B.G. Cavalheiro, J.P. Shah, G.W. Randolph, J.E. Medina, R.P. Tufano, M. Zafereo et al. Management of recurrent well-differentiated thyroid carcinoma in the neck: a comprehensive review. Cancers (Basel) 15, 923 (2023)
M.B. Amin, F.L. Greene, S.B. Edge, C.C. Compton, J.E. Gershenwald, R.K. Brookland et al. The eighth edition AJCC cancer staging manual: continuing to build a bridge from a population‐based to a more “personalized” approach to cancer staging. CA Cancer J. Clin. 67, 93–99 (2017)
R.M. Tuttle, H. Tala, J. Shah, R. Leboeuf, R. Ghossein, M. Gonen et al. Estimating risk of recurrence in differentiated thyroid cancer after total thyroidectomy and radioactive iodine remnant ablation: using response to therapy variables to modify the initial risk estimates predicted by the new American Thyroid Association staging system. Thyroid 20, 1341–1349 (2010)
M. Ora, A.H. Nazar, P. Mishra, S. Barai, A. Arya, P.K. Pradhan et al. Factors predicting the risk of biochemical incomplete response in well-differentiated thyroid cancer after total thyroidectomy. Nucl. Med. Commun 42, 1187–1194 (2021)
J. Ahn, E. Song, W.G. Kim, T.Y. Kim, W.B. Kim, Y.K. Shong et al. Long-term clinical outcomes of papillary thyroid carcinoma patients with biochemical incomplete response. Endocrine 67, 623–629 (2020)
A.K. Siraj, S.K. Parvathareddy, P. Pratheeshkumar, S.P. Divya, S.S. Al-Sobhi, F. Al-Dayel et al. PD-L1 is an independent prognostic marker in middle eastern PTC and its expression is upregulated by BRAFV600E mutation. Cancers (Basel) 13, 555 (2021)
R. Bu, A.K. Siraj, S.P. Divya, Y. Kong, S.K. Parvathareddy, M. Al‐Rasheed et al. Telomerase reverse transcriptase mutations are independent predictor of disease‐free survival in M iddle E astern papillary thyroid cancer. Int. J. Cancer 142, 2028–2039 (2018)
M. Steinschneider, J. Pitaro, S. Koren, Y. Mizrakli, C. Benbassat, L. Muallem Kalmovich, Differentiated thyroid cancer with biochemical incomplete response: clinico-pathological characteristics and long term disease outcomes. Cancers (Basel) 13, 5422 (2021)
F. Yu, X. Li, Y. Ji, J. Tan, G. Zhang, P. Wang et al. Delayed initial radioiodine adjuvant therapy does affect biochemical response in intermediate-to high-risk differentiated thyroid cancer. Front Endocrinol (Lausanne) 12, 743310 (2021)
F. Vaisman, D. Momesso, D.A. Bulzico, C.H. Pessoa, F. Dias, R. Corbo et al. Spontaneous remission in thyroid cancer patients after biochemical incomplete response to initial therapy. Clin. Endocrinol (Oxf) 77, 132–138 (2012)
F. Pacini, L. Agate, R. Elisei, M. Capezzone, C. Ceccarelli, F. Lippi et al. Outcome of differentiated thyroid cancer with detectable serum Tg and negative diagnostic 131I whole body scan: comparison of patients treated with high 131I activities versus untreated patients. J. Clin. Endocrinol. Metab. 86, 4092–4097 (2001)
C. Nascimento, I. Borget, A. Al Ghuzlan, D. Deandreis, L. Chami, J. Travagli et al. Persistent disease and recurrence in differentiated thyroid cancer patients with undetectable postoperative stimulated thyroglobulin level. Endocr. Relat. Cancer 18, R29–R40 (2011)
P.W. Rosario, A.C.M. Xavier, M.R. Calsolari, Value of postoperative thyroglobulin and ultrasonography for the indication of ablation and 131I activity in patients with thyroid cancer and low risk of recurrence. Thyroid 21, 49–53 (2011)
R.C. Webb, R.S. Howard, A. Stojadinovic, D.Y. Gaitonde, M.K. Wallace, J. Ahmed et al. The utility of serum thyroglobulin measurement at the time of remnant ablation for predicting disease-free status in patients with differentiated thyroid cancer: a meta-analysis involving 3947 patients. J. Clin. Endocrinol. Metab. 97, 2754–2763 (2012)
H. Li, Y.Q. Zhang, C. Wang, X. Zhang, X. Li, Y.S. Lin, Delayed initial radioiodine therapy related to incomplete response in low‐to intermediate‐risk differentiated thyroid cancer. Clin. Endocrinol. (Oxf) 88, 601–606 (2018)
R.S. Scheffel, A.B. Zanella, J.M. Dora, A.L. Maia, Timing of radioactive iodine administration does not influence outcomes in patients with differentiated thyroid carcinoma. Thyroid 26, 1623–1629 (2016)
M. Kim, M. Han, M.J. Jeon, W.G. Kim, I.J. Kim, J.S. Ryu et al. Impact of delayed radioiodine therapy in intermediate‐/high‐risk papillary thyroid carcinoma. Clin. Endocrinol. (Oxf) 91, 449–455 (2019)
A. Machens, S. Hauptmann, H. Dralle, Disparities between male and female patients with thyroid cancers: sex difference or gender divide? Clin Endocrinol (Oxf) 65, 500–505 (2006)
N. Nilubol, L. Zhang, E. Kebebew, Multivariate analysis of the relationship between male sex, disease-specific survival, and features of tumor aggressiveness in thyroid cancer of follicular cell origin. Thyroid 23, 695–702 (2013)
J. Jonklaas, G. Nogueras-Gonzalez, M. Munsell, D. Litofsky, K. Ain, S. Bigos et al. The impact of age and gender on papillary thyroid cancer survival. J. Clin. Endocrinol. Metab. 97, E878–E887 (2012)
A.K. Siraj, S.K. Parvathareddy, P. Annaiyappanaidu, N. Siraj, S.S. Al-Sobhi, F. Al-Dayel et al. Male sex is an independent predictor of recurrence-free survival in middle Eastern papillary thyroid carcinoma. Front Endocrinol (Lausanne) 13, 292 (2022)
M.A. Adam, J. Pura, P. Goffredo, M.A. Dinan, S.D. Reed, R.P. Scheri et al. Presence and number of lymph node metastases are associated with compromised survival for patients younger than age 45 years with papillary thyroid cancer. J. Clin. Oncol. 33, 2370–2375 (2015)
V. Zaydfudim, I.D. Feurer, M.R. Griffin, J.E. Phay, The impact of lymph node involvement on survival in patients with papillary and follicular thyroid carcinoma. Surgery 144, 1070–1078 (2008)
C. Yun, J. Xiao, J. Cao, C. Shao, L. Wang, W. Zhang et al. Lymph node metastases> 5 and metastatic lymph node ratio> 0.30 of differentiated thyroid cancer predict response to radioactive iodine. Cancer Med. 10, 7610–7619 (2021)
Acknowledgements
We would like to thank Kaleem Iqbal and Nabil Siraj for their technical assistance.
Author contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Sandeep Kumar Parvathareddy, Abdul K. Siraj, Saeeda O. Ahmed, Padmanaban Annaiyappanaidu,Maha Al-Rasheed, Wael Al-Haqawi and Zeeshan Qadri. Clinical assistance was provided by Saif S. Al-Sobhiand Fouad Al-Dayel. The first draft of the manuscript was written by Khawla S. Al-Kuraya and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethics
The Institutional Review Board of King Faisal Specialist Hospital and Research Centre (Riyadh, Saudi Arabia)approved this study and since only retrospective patient data were used, the Research Advisory Council (RAC) provided waiver of consent under project RAC # 221 1168 and # 2110 031. The study was conducted in accordance with the Declaration of Helsinki.All cases were de-identified, and all clinical-pathological data were accessed anonymously.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Parvathareddy, S.K., Siraj, A.K., Ahmed, S.O. et al. Predicting factors and clinical outcome of biochemical incomplete response in middle eastern differentiated thyroid carcinoma. Endocrine (2024). https://doi.org/10.1007/s12020-024-03844-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12020-024-03844-x