Abstract
Purpose
Familial hypercholesterolemia (FH) is one of the most common inherited diseases characterized by elevated LDL-cholesterol levels, leading to early-onset atherosclerosis. While the association between FH and coronary and carotid artery disease is well-established, its association with peripheral artery disease (PAD) is less robust. This systematic review aims at exploring existing evidence on PAD prevalence and incidence in FH individuals.
Methods
A comprehensive search was conducted on MEDLINE and Embase databases, for studies published between January 2013 and December 2023, evaluating prevalence and incidence of PAD in FH patients. Literature reviews, case reports, responses to editors and non-English language articles were excluded.
Results
The initial research provided 53 results. After article screening, 28 articles were fully reviewed and 24 were finally included in the analysis. Among these, 19 reported PAD prevalence, while 5 PAD incidence over a mean follow-up time of 8.7 years. PAD prevalence and incidence ranged from 0.3 to 60% and from 0.5 to 4.2% respectively, probably reflecting the heterogeneity in PAD definition criteria.
Conclusion
This systematic review sheds light on the limited number of studies on PAD in FH patients. Particularly, considering the potential positive effects of newly available lipid-lowering strategies on PAD outcomes, addressing this research gap is pivotal for a more comprehensive understanding of peripheral vascular manifestations in FH patients and for optimal management of this population.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Familial hypercholesterolemia (FH) is one of the most common autosomal dominant genetic diseases, characterized by an increased exposure to elevated low-density lipoprotein cholesterol (LDL-C) levels that result in a propensity for early atherosclerotic cardiovascular disease (ACVD) [1].
It is caused by mutations in genes involved in LDL-C catabolism, such as functional mutation in the LDL receptor (LDLR), apolipoprotein B (APOB) or proprotein convertase subtilisin kexin-9 (PCSK9) [2, 3]. The prevalence of FH in the general population is about 1 in 300, affecting about 14 million people worldwide [4, 5].
The association between FH and coronary artery disease is widely described; the prevalence of clinical FH among patients with premature acute myocardial infarction (MI) (age ≤50 years) is approximately 30 times higher than in the general population and cardiovascular risk factors prevalence increases with age of diagnosis [1, 6].
Peripheral artery disease (PAD) is a prevalent yet underdiagnosed condition characterized by a wide spectrum of clinical manifestations [7]. Only 10 to 30% of patients with PAD present with classical symptoms of intermittent claudication, while the remaining present with non-specific symptoms or are asymptomatic [7]. It is estimated to affect over 200 million adults in the world and its burden has continuously increased since 1990 [8]. Diabetes, cigarette smoking and hypertension have been associated with an increased risk of PAD, whereas the relationship between dyslipidemia and PAD is rather multifaceted, with features of atherogenic dyslipidemia being more related to PAD than simple LDL-C levels [9, 10].
Methodologies for PAD diagnosis include ankle-brachial index (ABI), segmental limb pressure evaluation, treadmill testing, pulse-volume waveform analysis and imaging techniques (such as duplex ultrasonography, CT angiography and MR angiography) [7].
The first-line noninvasive diagnostic method for PAD is the ABI, with sensitivity and specificity ranging from 61 to 73% and from 83 to 96% respectively [7]. However, sensitivity might decrease and ABI might be falsely high in patients with advanced age, diabetes or chronic kidney disease, because of the higher probability of medial artery calcification [7]. In these particular cases, other tests such as the toe-brachial index (TBI) may be considered [7].
Data from the largest international registry of FH suggest that PAD is found in about 5% of FH population [1], however it is unclear whether diagnosis is based on symptoms, imaging, or other methods like ankle brachial index (ABI). In addition to the healthcare expenses linked to addressing complications related to PAD, a primary concern is the insufficient awareness of the condition both among patients and practitioners [1].
This systematic review aims to provide a comprehensive assessment and investigate the prevalence and incidence of PAD in individuals living with FH.
Methods
Search strategy
The study protocol was registered on PROSPERO (registration number CRD42023476568) in accordance with PRISMA guidelines [11].
The online MEDLINE and Embase databases were searched for medical subject heading (MeSH) and keywords related to PAD and FH (see Supplementary Methods 1). These searches were supplemented by examining reference lists of included studies, reviews, and meta-analyses.
Studies selection
Inclusion criteria were as follows: (1) randomized controlled trial (RCTs) and/or observational studies; (2) evaluating the prevalence of peripheral artery disease (PAD) in patients with familial hypercholesterolemia (FH) published between 1 January 2013 and 14 November 2023.
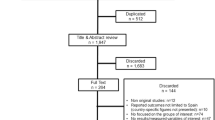
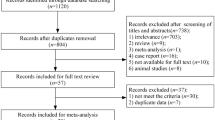
Literature reviews, case reports, responses to editors and non-English language articles were excluded. The initial research provided 53 results. After article screening, 28 articles were fully reviewed and 24 articles referring to heterozygous familial hypercholesterolemia (HeFH) were included in the final analysis (Supplementary Fig. 1). Among these articles, there were 9 cross-sectional studies, 7 retrospective studies, 6 prospective studies and 2 pooled randomized controlled trials (RCTs).
Data extraction
Data extracted from selected articles included: year of publication, type of study, journal, first author, recruitment time or follow-up, population (number of patients), gender, age, FH diagnosis (molecular or clinical), ethnicity, country, baseline lipid profile, comorbidities (hypertension, diabetes, smoking), family history of premature cardiovascular disease, ACVD status (primary or secondary prevention), lipid lowering therapy, criteria for PAD definition, study outcome and results, reported prevalence or incidence of PAD, coronary artery disease (acute coronary syndrome or stable angina) and carotid artery disease (stenosis or stroke).
Results
The main information extracted from the reviewed articles is summarized in Table 1 and Table 2. Nineteen studies reported PAD prevalence (Table 1) while 5 studies reported PAD incidence during the follow-up time (Table 2). The age distribution of study populations ranged from 40.3 to 62.7 years. Reported PAD prevalence ranged from 0.7 and 60% in cross sectional studies, from 0.3 to 15.6% in retrospective studies and from 1.4 and 12.4% in prospective studies. The 2 RCTs reported a PAD prevalence of 5.7 and 6%. Reported PAD incidence varied from 0.5 to 4.2% during a mean observation time of 8.7 years.
PAD prevalence and incidence (according to definition criteria)
In the reviewed literature, PAD definition criteria were heterogeneous.
Prevalence
A subset of studies (n = 4) defined PAD with more stringent criteria (i.e. as intermittent claudication with either ABI < 0.9 or evidence of stenosis >50% at imaging; ABI ≤ 0.9 alone; intermittent claudication or ABI < 0.9 or stenosis >50% at imaging; claudication alone) and reported the lowest prevalence (1.3–6%).
Two studies defined PAD according to the International Classification of Diseases (ICD) system (Table 1 and Supplementary Table 3), reporting a PAD prevalence of 12.4 and 15.6%.
Three studies defined PAD according to wider criteria (evidence of femoral plaque, IMT > 1.5 mm). Among these studies, PAD prevalence was similar and ranged between 54.5 and 60%.
Finally, a substantial subset of articles (n = 10) did not explicitly provide a specific definition for PAD. Among these articles, PAD prevalence showed the highest variability (ranging from 0.3 and 13%).
Interestingly, the majority of studies aiming at assessing PAD prevalence as a primary outcome reported overall lower prevalence rates compared to those for which prevalence assessment was not a primary outcome.
Incidence
Among the 5 studies evaluating PAD incidence, one defined PAD as intermittent claudication or ABI < 0.9 or stenosis >50%: in this study the incidence of PAD was as low as 0.8% during the observation time. One study relied on the International Classification of Diseases (ICD) system (Table 2 and Supplementary Table 3) for PAD definition and reported an incidence of hospitalizations due to PAD of 1.2%.
One study, defining PAD as arterial embolism, thrombosis, occlusion or stenosis, reported an incidence rate of 1.5%.
Two studies did not specify PAD definition criteria, and reported an incidence of 0.5 and 4.2%.
In this case, a lower incidence was reported in studies for which incidence assessment was not the primary outcome.
FH definition
The majority of studies relied on the Dutch Lipid Clinic Network (DLCN) criteria and genetic analysis, either individually or in combination. A minority of studies employed alternative clinical criteria for defining FH, such as the Simon Broome criteria, MEDPED criteria, or the Japan Atherosclerosis Society guidelines.
Moreover, clinical features that accurately characterize the FH population (including detailed lipid-lowering treatment, lipid profile and category of cardiovascular prevention) were often lacking in the analyzed studies, and are shown in the Supplementary Tables 1 and 2.
PAD and cardiovascular risk factors
All studies evaluating PAD prevalence also reported cardiovascular risk factors prevalence including diabetes, hypertension and cigarette smoking. Diabetes prevalence ranged between 2.4 and 28.6%, with the exception of one study in which patients with diabetes were not included. Prevalence of hypertension and smoking ranged between 12 and 66.4% and between 11.6 and 45.1%, respectively. Interestingly, PAD did not vary significantly according to reported prevalence of other cardiovascular risk factors.
Four studies investigating PAD incidence reported the prevalence of other cardiovascular risk factors. Prevalence of diabetes, hypertension and smoking ranged between 1.8 and 6.2%, 5.6 and 28% and 16 and 81.7%, respectively.
Prevalence of these cardiovascular risk factors did not seem to change significantly among the studies (with the exception of one study defining smokers as both current and past smokers, thus reporting a higher smoking percentage). As outlined before, prevalence of cardiovascular risk factors was not apparently associated with a higher incidence of PAD.
Coronary and carotid involvement
Among the 19 studies evaluating PAD prevalence, 15 investigated both coronary and carotid vascular involvement, 2 evaluated coronary involvement only, and 1 evaluated carotid involvement only. Coronary and carotid disease prevalence ranged between 9.6 and 95% and between 0 and 70.2%, respectively. In the majority of studies, prevalence of coronary and carotid diseases was higher in studies reporting a higher PAD prevalence. Four of the 5 studies investigating PAD incidence assessed incidence of coronary and carotid vascular disease as well. Coronary artery disease incidence varied from 8.2 to 25%, while carotid artery disease incidence varied from 1.4 and 5.4% during the observation time. Studies reporting the highest incidence of PAD reported the highest incidence of coronary and carotid disease as well.
Discussion
In this review, we analyzed 24 articles reporting PAD prevalence or incidence among FH patients. Based on definition, PAD prevalence ranged from 0.3 to 60%, with the lowest variability among prospective studies and those defining PAD with more stringent criteria. The incidence of PAD during follow-up varied from 0.5 to 4.2%. Furthermore, PAD definition was not specified in 12 out of 24 articles analyzed.
Coronary and carotid involvement showed high variability in prevalence (9.6–95 and 0–70.2%, respectively) and incidence (8.2–25 and 1.4–5.4%, respectively). This high variability may reflect the fact that the majority of studies were including both subjects in primary and secondary prevention.
Regarding FH diagnosis, most studies used the DLCN criteria and genetic analysis, and detailed data on lipid-lowering treatments and lipid profiles were often missing.
Data regarding PAD epidemiology among FH patients is sparse as compared to other atherosclerotic territories involved [1]. A recent meta-analysis found a PAD prevalence of 5.2% worldwide, with a prevalence of 6.8% in Europe [1]. However, our study shows extreme variability in the prevalence estimation of PAD in FH.
The observed heterogeneity might be attributable to multiple factors. Firstly, PAD encompasses a wide range of manifestations, ranging from lower limb amputation to asymptomatic subclinical lower-limb atherosclerosis, defined by an ankle-brachial index (ABI) of 0.90 or less. Though these patients are at high risk of cardiovascular events and mortality [12], PAD is often underdiagnosed even in the general population [13]. Thus, estimating its prevalence could be difficult due to differences in the investigated populations and to the lack of a uniform PAD definition. This is clearly reflected by this systematic review. As a matter of fact, the three studies with the highest PAD prevalence (54.5, 56 and 60%) included femoral plaques in their PAD definition [14,15,16]; by excluding these three studies, the prevalence would range between 0.3 and 15.6% with the lowest rates reported mainly in the studies defining PAD as ABI < 0.9. Secondly, apart from PAD definition, for studies evaluating the prevalence of PAD the lower was the estimated prevalence the higher was the quality of the study design, and vice versa. Interestingly, most of the analyzed studies were conducted by clinicians alone, and only two studies were conducted by clinicians together with vascular surgeons. This may contribute to the variability of the outcome definition and account for the selection bias of the studied population. Familial hypercholesterolemia unawareness among health professionals not dedicated to lipid disorders is still quite frequent and may account for the underdiagnosis of this condition in subjects undergoing vascular procedures, highlighting the need for a multidisciplinary counseling. Thirdly, it must be remembered that about 50% of subjects with PAD are asymptomatic. Moreover, when studies evaluate PAD according to ICD definition, it must be acknowledged that the diagnostic power is reduced, even more in asymptomatic subjects.
Finally, age is one of the major PAD risk factors [9]. One systematic review estimated a global prevalence of PAD defined by an ABI of 0.90 or less in people aged 25 years and older of 5.56% (95% CI: 3.7–8.55) [17]. Another recent published metanalysis found a global prevalence of 9.7% (95% CI: 7.1–12.4) in adults mostly >40 years old, with age, smoking status, hypertension, diabetes and hypercholesterolemia ≥200 mg/dL being significantly associated risk factors [9]. In the light of these considerations, PAD prevalence among FH patients seems to be similar to the one reported in the general population. This is significant, considering that the FH population is typically younger and less exposed to cardiovascular risk factors than the general population. Lower extremity PAD is prevalent among people aged 50 years and older in the general population [9]. Though exposed to high LDL-C levels since birth, FH subjects are younger than those usually assessed in the general population. Still, age-adjusted comparisons seem to find higher PAD prevalence among FH patients than non FH patients [18, 19], suggesting a premature PAD incidence among FH patients despite the variability of PAD prevalence/incidence we analyzed. In our analysis, we could not investigate the weight of well-recognized risk factors for PAD (like smoking and diabetes), since these risk factors were not uniformly and systematically reported [20,21,22]. However, PAD did not seem to vary significantly according to reported prevalence of other cardiovascular risk factors when available. Familial hypercholesterolemia is clearly associated with an increased risk of coronary heart disease, as largely reported in the literature and as shown by most extreme clinical presentations, as in homozygous FH [23, 24]. However, the prevalence of common cardiovascular risk factors in this population is associated with a higher cardiovascular risk. Equations aimed at stratifying cardiovascular risk in FH clearly show an improved CV risk prediction when classical cardiovascular risk factors are taken into account [25,26,27,28]. Regarding ischemic stroke, a recent study based on the Copenhagen general population failed to show an association between FH and ischemic stroke [29], though evidence from RCTs showed a benefit on stroke from LDL-C reduction with statins [30, 31]. This illustrates the complexity of the link between LDL-C and its consequences depending on the anatomical territories assessed. Regarding PAD, increasing evidence based on Mendelian randomization studies [32] and effects from both statins and PCSK9 inhibitors on vascular outcomes tends to demonstrate a causative role of LDL-C [33,34,35]. Furthermore, the Progression of Early Subclinical Atherosclerosis (PESA) study has shown that lower limbs atherosclerosis in asymptomatic middle aged individuals highlight subclinical atherosclerotic burden in subjects otherwise considered at low risk [36], and femoral atherosclerosis was the best predictor of atherosclerosis in other vascular districts.
Conclusions
The scarcity and sparsity of data available in the literature underlines the critical need for improved, more standardized and better-defined screening of PAD in patients with FH. A systematic approach in identifying PAD, either symptomatic or asymptomatic, with more uniform criteria could be useful since patients with polyvascular disease are at particularly high risk for future cardiovascular events and warrant aggressive management of standard atherosclerotic risk factors. At present, current guidelines do not recommend screening strategies for PAD, in patients without signs or symptoms of claudication. Future prospective studies evaluating PAD in subjects with FH could provide valuable insights into their long-term cardiovascular outcomes and tailor strategies of novel lipid-lowering agents such as PCSK-9 inhibitors for treatment of these high-risk individuals, in particular in the context of improved life- expectancy.
References
EAS Familial Hypercholesterolaemia Studies Collaboration (FHSC), Global perspective of familial hypercholesterolaemia: a cross-sectional study from the EAS Familial Hypercholesterolaemia Studies Collaboration (FHSC). Lancet 398, 1713–1725 (2021). https://doi.org/10.1016/S0140-6736(21)01122-3
M.A. Austin, C.M. Hutter, R.L. Zimmern, S.E. Humphries, Genetic causes of monogenic heterozygous familial hypercholesterolemia: a HuGE prevalence review. Am. J. Epidemiol. 160, 407–420 (2004). https://doi.org/10.1093/aje/kwh236
B.G. Nordestgaard, M.J. Chapman, S.E. Humphries, H.N. Ginsberg, L. Masana, O.S. Descamps, O. Wiklund, R.A. Hegele, F.J. Raal, J.C. Defesche, A. Wiegman, R.D. Santos, G.F. Watts, K.G. Parhofer, G.K. Hovingh, P.T. Kovanen, C. Boileau, M. Averna, J. Borén, E. Bruckert, A.L. Catapano, J.A. Kuivenhoven, P. Pajukanta, K. Ray, A.F. Stalenhoef, E. Stroes, M.R. Taskinen, A. Tybjærg-Hansen; European Atherosclerosis Society Consensus Panel, Familial hypercholesterolaemia is underdiagnosed and undertreated in the general population: guidance for clinicians to prevent coronary heart disease: consensus statement of the European Atherosclerosis Society. Eur. Heart J. 34, 3478–3490a (2013). https://doi.org/10.1093/eurheartj/eht273
M. Benn, G.F. Watts, A. Tybjaerg-Hansen, B.G. Nordestgaard, Familial hypercholesterolemia in the danish general population: prevalence, coronary artery disease, and cholesterol-lowering medication. J. Clin. Endocrinol. Metab. 97, 3956–3964 (2012). https://doi.org/10.1210/jc.2012-1563
P. Hu, K.I. Dharmayat, C.A.T. Stevens, M.T.A. Sharabiani, R.S. Jones, G.F. Watts, J. Genest, K.K. Ray, A.J. Vallejo-Vaz, Prevalence of Familial Hypercholesterolemia Among the General Population and Patients With Atherosclerotic Cardiovascular Disease: A Systematic Review and Meta-Analysis. Circulation 141, 1742–1759 (2020). https://doi.org/10.1161/CIRCULATIONAHA.119.044795
M. Hauguel-Moreau, V. Aïdan, H. Hergault, A. Beauchet, M. Pépin, G. Prati, R. Pillière, M. Ouadahi, L. Josseran, C. Rodon, J.P. Rabès, P. Charron, O. Dubourg, Z. Massy, N. Mansencal, Prevalence of familial hypercholesterolaemia in patients presenting with premature acute coronary syndrome. Arch. Cardiovasc Dis. 115, 87–95 (2022). https://doi.org/10.1016/j.acvd.2021.11.005
M.H. Criqui, K. Matsushita, V. Aboyans, C.N. Hess, C.W. Hicks, T.W. Kwan, M.M. McDermott, S. Misra, F. Ujueta; American Heart Association Council on Epidemiology and Prevention; Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Cardiovascular Radiology and Intervention; Council on Lifestyle and Cardiometabolic Health; Council on Peripheral Vascular Disease; and Stroke Council, Lower Extremity Peripheral Artery Disease: Contemporary Epidemiology, Management Gaps, and Future Directions: A Scientific Statement From the American Heart Association. Circulation 144, e171–e191 (2021). https://doi.org/10.1161/CIR.0000000000001005
G.A. Roth, G.A. Mensah, C.O. Johnson, G. Addolorato, E. Ammirati, L.M. Baddour, N.C. Barengo, A.Z. Beaton, E.J. Benjamin, C.P. Benziger, A. Bonny, M. Brauer, M. Brodmann, T.J. Cahill, J. Carapetis, A.L. Catapano, S.S. Chugh, L.T. Cooper, J. Coresh, M. Criqui, N. DeCleene, K.A. Eagle, S. Emmons-Bell, V.L. Feigin, J. Fernández-Solà, G. Fowkes, E. Gakidou, S.M. Grundy, F.J. He, G. Howard, F. Hu, L. Inker, G. Karthikeyan, N. Kassebaum, W. Koroshetz, C. Lavie, D. Lloyd-Jones, H.S. Lu, A. Mirijello, A.M. Temesgen, A. Mokdad, A.E. Moran, P. Muntner, J. Narula, B. Neal, M. Ntsekhe, G. Moraes de Oliveira, C. Otto, M. Owolabi, M. Pratt, S. Rajagopalan, M. Reitsma, A.L.P. Ribeiro, N. Rigotti, A. Rodgers, C. Sable, S. Shakil, K. Sliwa-Hahnle, B. Stark, J. Sundström, P. Timpel, I.M. Tleyjeh, M. Valgimigli, T. Vos, P.K. Whelton, M. Yacoub, L. Zuhlke, C. Murray, V. Fuster; GBD-NHLBI-JACC Global Burden of Cardiovascular Diseases Writing Group, Global Burden of Cardiovascular Diseases and Risk Factors, 1990-2019: Update From the GBD 2019 Study. J. Am. Coll. Cardiol. 76, 2982–3021 (2020). https://doi.org/10.1016/j.jacc.2020.11.010
C. Adou, J. Magne, N. Gazere, M. Aouida, L. Chastaingt, V. Aboyans. Global Epidemiology of Lower Extremity Artery Disease in the 21st Century (2000-2021): a Systematic Review and Meta-analysis. Eur. J. Prev. Cardiol. zwad381 (2023). https://doi.org/10.1093/eurjpc/zwad381
A.W. Aday, P.R. Lawler, N.R. Cook, P.M. Ridker, S. Mora, A.D. Pradhan, Lipoprotein Particle Profiles, Standard Lipids, and Peripheral Artery Disease Incidence. Circulation 138, 2330–2341 (2018). https://doi.org/10.1161/CIRCULATIONAHA.118.035432
D. Moher, A. Liberati, J. Tetzlaff, D.G. Altman, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 6, e1000097 (2009). https://doi.org/10.1371/journal.pmed.1000097
Ankle Brachial Index Collaboration, F.G. Fowkes, G.D. Murray, I. Butcher, C.L. Heald, R.J. Lee, L.E. Chambless, A.R. Folsom, A.T. Hirsch, M. Dramaix, G. deBacker, J.C. Wautrecht, M. Kornitzer, A.B. Newman, M. Cushman, K. Sutton-Tyrrell, F.G. Fowkes, A.J. Lee, J.F. Price, R.B. d’Agostino, J.M. Murabito, P.E. Norman, K. Jamrozik, J.D. Curb, K.H. Masaki, B.L. Rodríguez, J.M. Dekker, L.M. Bouter, R.J. Heine, G. Nijpels, C.D. Stehouwer, L. Ferrucci, M.M. McDermott, H.E. Stoffers, J.D. Hooi, J.A. Knottnerus, M. Ogren, B. Hedblad, J.C. Witteman, M.M. Breteler, M.G. Hunink, A. Hofman, M.H. Criqui, R.D. Langer, A. Fronek, W.R. Hiatt, R. Hamman, H.E. Resnick, J. Guralnik, M.M. McDermott, Ankle brachial index combined with Framingham Risk Score to predict cardiovascular events and mortality: a meta-analysis. JAMA 300, 197–208 (2008). https://doi.org/10.1001/jama.300.2.197
A. Mehta, D.S. Dhindsa, A. Hooda, A. Nayak, C.S. Massad, B. Rao, L.F. Makue, R.R. Rajani, O. Alabi, A.A. Quyyumi, G.A. Escobar, B.J. Wells, L.S. Sperling, Premature atherosclerotic peripheral artery disease: An underrecognized and undertreated disorder with a rising global prevalence. Trends Cardiovasc. Med. 31, 351–358 (2021). https://doi.org/10.1016/j.tcm.2020.06.005
Y.X. Cao, H.H. Liu, D. Sun, J.L. Jin, R.X. Xu, Y.L. Guo, N.Q. Wu, C.G. Zhu, S. Li, Y. Zhang, J. Sun, J.J. Li, The different relations of PCSK9 and Lp(a) to the presence and severity of atherosclerotic lesions in patients with familial hypercholesterolemia. Atherosclerosis 277, 7–14 (2018). https://doi.org/10.1016/j.atherosclerosis.2018.07.030
A. Mattina, A. Giammanco, P. Giral, D. Rosenbaum, A. Carrié, P. Cluzel, A. Redheuil, R. Bittar, S. Béliard, D. Noto, A. Quartarone, M. Averna, É. Bruckert, A. Gallo, Polyvascular subclinical atherosclerosis in familial hypercholesterolemia: The role of cholesterol burden and gender. Nutr. Metab. Cardiovasc. Dis. 29, 1068–1076 (2019). https://doi.org/10.1016/j.numecd.2019.06.015
A.J. Amor, E. Ortega, V. Perea, M. Cofán, A. Sala-Vila, I. Nuñez, R. Gilabert, E. Ros, Relationship Between Total Serum Bilirubin Levels and Carotid and Femoral Atherosclerosis in Familial Dyslipidemia. Arterioscler Thromb. Vasc. Biol. 37, 2356–2363 (2017). https://doi.org/10.1161/ATVBAHA.117.310071
P. Song, D. Rudan, Y. Zhu, F.J.I. Fowkes, K. Rahimi, F.G.R. Fowkes, I. Rudan, Global, regional, and national prevalence and risk factors for peripheral artery disease in 2015: an updated systematic review and analysis. Lancet Glob. Health 7, e1020–e1030 (2019). https://doi.org/10.1016/S2214-109X(19)30255-4
C. Pereira, M.H. Miname, M.R. Makdisse, C. Watanabe, A.E. Pesaro, C.E. Jannes, R. Kalil Filho, A.C. Pereira, R.D. Santos, Peripheral arterial disease in heterozygous familial hypercholesterolemia. Atherosclerosis 242, 174–178 (2015). https://doi.org/10.1016/j.atherosclerosis.2015.07.022
L.J. Mundal, A. Hovland, J. Igland, M. Vetrhus, M.B. Veierød, K.B. Holven, M.P. Bogsrud, G.S. Tell, T.P. Leren, K. Retterstøl, Increased risk of peripheral artery disease in persons with familial hypercholesterolaemia: a prospective registry study. Eur. J. Prev. Cardiol. 28, e11–e13 (2022). https://doi.org/10.1093/eurjpc/zwaa024
E.M. Willigendael, J.A. Teijink, M.L. Bartelink, B.W. Kuiken, J. Boiten, F.L. Moll, H.R. Büller, M.H. Prins, Influence of smoking on incidence and prevalence of peripheral arterial disease. J. Vasc. Surg. 40, 1158–1165 (2004). https://doi.org/10.1016/j.jvs.2004.08.049
L. Lu, D.F. Mackay, J.P. Pell, Meta-analysis of the association between cigarette smoking and peripheral arterial disease. Heart 100, 414–423 (2014). https://doi.org/10.1136/heartjnl-2013-304082
S.P. Marso, W.R. Hiatt, Peripheral arterial disease in patients with diabetes. J. Am. Coll. Cardiol. 47, 921–929 (2006). https://doi.org/10.1016/j.jacc.2005.09.065
E. Bruckert, O. Kalmykova, R. Bittar, V. Carreau, S. Béliard, S. Saheb, D. Rosenbaum, D. Bonnefont-Rousselot, D. Thomas, C. Emery, B. Khoshnood, A. Carrié, Long-term outcome in 53 patients with homozygous familial hypercholesterolaemia in a single centre in France. Atherosclerosis 257, 130–137 (2017). https://doi.org/10.1016/j.atherosclerosis.2017.01.015
G.R. Thompson, D.J. Blom, A.D. Marais, M. Seed, G.J. Pilcher, F.J. Raal, Survival in homozygous familial hypercholesterolaemia is determined by the on-treatment level of serum cholesterol. Eur. Heart J. 39, 1162–1168 (2018). https://doi.org/10.1093/eurheartj/ehx317
L. Pérez de Isla, R. Alonso, N. Mata, C. Fernández-Pérez, O. Muñiz, J.L. Díaz-Díaz, A. Saltijeral, F. Fuentes-Jiménez, R. de Andrés, D. Zambón, M. Piedecausa, J.M. Cepeda, M. Mauri, J. Galiana, Á. Brea, J.F. Sanchez Muñoz-Torrero, T. Padró, R. Argueso, J.P. Miramontes-González, L. Badimón, R.D. Santos, G.F. Watts, P. Mata, Predicting Cardiovascular Events in Familial Hypercholesterolemia: The SAFEHEART Registry (Spanish Familial Hypercholesterolemia Cohort Study). Circulation 135, 2133–2144 (2017). https://doi.org/10.1161/CIRCULATIONAHA.116.024541
A. Gallo, S. Charriere, A. Vimont, M.J. Chapman, D. Angoulvant, F. Boccara, B. Cariou, V. Carreau, A. Carrié, E. Bruckert, S. Béliard; French REgistry of Familial hypERCHOLesterolemia (REFERCHOL) investigators, SAFEHEART risk-equation and cholesterol-year-score are powerful predictors of cardiovascular events in French patients with familial hypercholesterolemia. Atherosclerosis 306, 41–49 (2020). https://doi.org/10.1016/j.atherosclerosis.2020.06.011
A. Gallo, L. Pérez de Isla, S. Charrière, A. Vimont, R. Alonso, O. Muñiz-Grijalvo, J.L. Díaz-Díaz, D. Zambón, P. Moulin, E. Bruckert, P. Mata, S. Béliard; REFERCHOL and SAFEHEART Investigators, The Added Value of Coronary Calcium Score in Predicting Cardiovascular Events in Familial Hypercholesterolemia. JACC Cardiovasc. Imaging 14, 2414–2424 (2021). https://doi.org/10.1016/j.jcmg.2021.06.011
M. Paquette, B. Cariou, S. Bernard, R.A. Hegele, A. Gallo, J. Genest, M. Trinder, L.R. Brunham, S. Béliard, A. Baass. Increased FH-Risk-Score and Diabetes Are Cardiovascular Risk Equivalents in Heterozygous Familial Hypercholesterolemia. Arterioscler. Thromb. Vasc. Biol. (2023). https://doi.org/10.1161/ATVBAHA.123.319957
S. Beheshti, C.M. Madsen, A. Varbo, M. Benn, B.G. Nordestgaard, Relationship of Familial Hypercholesterolemia and High Low-Density Lipoprotein Cholesterol to Ischemic Stroke: Copenhagen General Population Study. Circulation 138, 578–589 (2018). https://doi.org/10.1161/CIRCULATIONAHA.118.033470
P. Amarenco, J. Bogousslavsky, A. Callahan 3rd, L.B. Goldstein, M. Hennerici, A.E. Rudolph, H. Sillesen, L. Simunovic, M. Szarek, K.M. Welch, J.A. Zivin, Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) Investigators. High-dose atorvastatin after stroke or transient ischemic attack. N. Engl. J. Med. 355, 549–559 (2006). https://doi.org/10.1056/NEJMoa061894
Cholesterol Treatment Trialists’ (CTT) Collaborators, B. Mihaylova, J. Emberson, L. Blackwell, A. Keech, J. Simes, E.H. Barnes, M. Voysey, A. Gray, R. Collins, C. Baigent, The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: meta-analysis of individual data from 27 randomised trials. Lancet 380, 581–590 (2012). https://doi.org/10.1016/S0140-6736(12)60367-5
F. Emanuelsson, M. Benn, LDL-Cholesterol versus Glucose in Microvascular and Macrovascular Disease. Clin. Chem. 67, 167–182 (2021). https://doi.org/10.1093/clinchem/hvaa242
Heart Protection Study Collaborative Group, Randomized trial of the effects of cholesterol-lowering with simvastatin on peripheral vascular and other major vascular outcomes in 20,536 people with peripheral arterial disease and other high-risk conditions. J. Vasc. Surg. 45, 645–654 (2007). https://doi.org/10.1016/j.jvs.2006.12.054
M.P. Bonaca, P. Nault, R.P. Giugliano, A.C. Keech, A.L. Pineda, E. Kanevsky, J. Kuder, S.A. Murphy, J.W. Jukema, B.S. Lewis, L. Tokgozoglu, R. Somaratne, P.S. Sever, T.R. Pedersen, M.S. Sabatine, Low-Density Lipoprotein Cholesterol Lowering With Evolocumab and Outcomes in Patients With Peripheral Artery Disease: Insights From the FOURIER Trial (Further Cardiovascular Outcomes Research With PCSK9 Inhibition in Subjects With Elevated Risk). Circulation 137, 338–350 (2018). https://doi.org/10.1161/CIRCULATIONAHA.117.032235
D.J. Kumbhani, P.G. Steg, C.P. Cannon, K.A. Eagle, S.C. Smith Jr, S. Goto, E.M. Ohman, Y. Elbez, P. Sritara, I. Baumgartner, S. Banerjee, M.A. Creager, D.L. Bhatt; REACH Registry Investigators, Statin therapy and long-term adverse limb outcomes in patients with peripheral artery disease: insights from the REACH registry. Eur. Heart J. 35, 2864–2872 (2014). https://doi.org/10.1093/eurheartj/ehu080
L. Fernández-Friera, J.L. Peñalvo, A. Fernández-Ortiz, B. Ibañez, B. López-Melgar, M. Laclaustra, B. Oliva, A. Mocoroa, J. Mendiguren, V. Martínez de Vega, L. García, J. Molina, J. Sánchez-González, G. Guzmán, J.C. Alonso-Farto, E. Guallar, F. Civeira, H. Sillesen, S. Pocock, J.M. Ordovás, G. Sanz, L.J. Jiménez-Borreguero, V. Fuster, Prevalence, Vascular Distribution, and Multiterritorial Extent of Subclinical Atherosclerosis in a Middle-Aged Cohort: The PESA (Progression of Early Subclinical Atherosclerosis) Study. Circulation 131, 2104–2113 (2015). https://doi.org/10.1161/CIRCULATIONAHA.114.014310
L. Pérez de Isla, R. Alonso, N. Mata, A. Saltijeral, O. Muñiz, P. Rubio-Marin, J.L. Diaz-Diaz, F. Fuentes, R. de Andrés, D. Zambón, J. Galiana, M. Piedecausa, R. Aguado, D. Mosquera, J.I. Vidal, E. Ruiz, L. Manjón, M. Mauri, T. Padró, J.P. Miramontes, P. Mata; SAFEHEART Investigators, Coronary Heart Disease, Peripheral Arterial Disease, and Stroke in Familial Hypercholesterolaemia: Insights From the SAFEHEART Registry (Spanish Familial Hypercholesterolaemia Cohort Study). Arterioscler Thromb. Vasc. Biol. 36, 2004–2010 (2016). https://doi.org/10.1161/ATVBAHA.116.307514
S. Funabashi, Y. Kataoka, M. Hori, M. Ogura, T. Doi, T. Noguchi, M. Harada-Shiba, Characterization of Polyvascular Disease in Heterozygous Familial Hypercholesterolemia: Its Association With Circulating Lipoprotein(a) Levels. J. Am. Heart Assoc. 11, e025232 (2022). https://doi.org/10.1161/JAHA.121.025232
A.J. Vallejo-Vaz, C.J. Packard, B.A. Ference, R.D. Santos, J.J.P. Kastelein, E.A. Stein, A.L. Catapano, T.R. Pedersen, G.F. Watts, K.K. Ray, LDL-cholesterol lowering and clinical outcomes in hypercholesterolemic subjects with and without a familial hypercholesterolemia phenotype: Analysis from the secondary prevention 4S trial. Atherosclerosis 320, 1–9 (2021). https://doi.org/10.1016/j.atherosclerosis.2021.01.003
F. Emanuelsson, B.G. Nordestgaard, M. Benn, Familial Hypercholesterolemia and Risk of Peripheral Arterial Disease and Chronic Kidney Disease. J. Clin. Endocrinol. Metab. 103, 4491–4500 (2018). https://doi.org/10.1210/jc.2018-01058
L. Masana, A. Zamora, N. Plana, M. Comas-Cufí, M. Garcia-Gil, R. Martí-Lluch, A. Ponjoan, L. Alves-Cabratosa, R. Elosua, J. Marrugat, I.R. Dégano, R. Ramos, Incidence of Cardiovascular Disease in Patients with Familial Hypercholesterolemia Phenotype: Analysis of 5 Years Follow-Up of Real-World Data from More than 1.5 Million Patients. J. Clin. Med 8, 1080 (2019). https://doi.org/10.3390/jcm8071080
P.A. McCullough, C.M. Ballantyne, S.K. Sanganalmath, G. Langslet, S.J. Baum, P.K. Shah, A. Koren, J. Mandel, M.H. Davidson, Efficacy and Safety of Alirocumab in High-Risk Patients With Clinical Atherosclerotic Cardiovascular Disease and/or Heterozygous Familial Hypercholesterolemia (from 5 Placebo-Controlled ODYSSEY Trials). Am. J. Cardiol. 121, 940–948 (2018). https://doi.org/10.1016/j.amjcard.2017.12.040
W.T. Fonzar, F.A. Fonseca, H.A. Fonseca, T.P. Silva, A.A. Rodrigues, D. Teixeira, M.E. Ishimura, M.E. Coste, C.N. França, H.T. Bianco, M. Gidlund, R.L. Morais, C.A. Bittencourt, C.A. Fonzar, V.A. Sant’Anna, I.L. Maugeri, J.B. Pesquero, M.C. Izar, Atherosclerosis severity in patients with familial hypercholesterolemia: The role of T and B lymphocytes. Atheroscler 48, 27–36 (2022). https://doi.org/10.1016/j.athplu.2022.03.002
K. Al-Waili, K. Al-Rasadi, F. Zadjali, K. Al-Hashmi, S. Al-Mukhaini, M. Al-Kindi, H. Al-Sabti, A.T. Al-Hinai, H. Farhan, I. Al-Zakwani, Clinical and Genetic Characteristics of Familial Hypercholesterolemia at Sultan Qaboos University Hospital in Oman. Oman. Med. J. 35, e141 (2020). https://doi.org/10.5001/omj.2020.59
P. Anagnostis, C.V. Rizos, I. Skoumas, L. Rallidis, K. Tziomalos, E. Skalidis, V. Kotsis, M. Doumas, G. Kolovou, G. Sfikas, A. Garoufi, V. Lambadiari, I. Dima, E. Kiouri, D. Agapakis, E. Zacharis, C. Antza, V. Kolovou, C. Koumaras, G. Bantouvakis, G. Liamis, E.N. Liberopoulos, Association between lipoprotein(a) concentrations and atherosclerotic cardiovascular disease risk in patients with familial hypercholesterolemia: an analysis from the HELLAS-FH. Endocrine 76, 324–330 (2022). https://doi.org/10.1007/s12020-022-03013-y
B. Zafrir, A. Jubran, G. Lavie, D.A. Halon, M.Y. Flugelman, C. Shapira, Clinical Features and Gaps in the Management of Probable Familial Hypercholesterolemia and Cardiovascular Disease. Circ. J. 82, 218–223 (2017). https://doi.org/10.1253/circj.CJ-17-0392
P.B. Duell, S.S. Gidding, R.L. Andersen, T. Knickelbine, L. Anderson, E. Gianos, P. Shrader, I. Kindt, E.C. O’Brien, D. McCann, L.C. Hemphill, C.D. Ahmed, S.S. Martin, J.A. Larry, Z.S. Ahmad, I.J. Kullo, J.A. Underberg, J. Guyton, P. Thompson, K. Wilemon, M.T. Roe, D.J. Rader, M. Cuchel, M.F. Linton, M.D. Shapiro, P.M. Moriarty, J.W. Knowles, Longitudinal low density lipoprotein cholesterol goal achievement and cardiovascular outcomes among adult patients with familial hypercholesterolemia: The CASCADE FH registry. Atherosclerosis 289, 85–93 (2019). https://doi.org/10.1016/j.atherosclerosis.2019.08.007
A. Matta, V. Bongard, F. Bouisset, D. Taraszkiewicz, J.P. Rabès, J. Ferrières, Real-World Efficacy of Proprotein Convertase Subtilisin/Kexin Type 9 Inhibitors (PCSK9i) in Heterozygous Familial Hypercholesterolemia Patients Referred for Lipoprotein Apheresis. Med. Sci. Monit. 27, e928784 (2021). https://doi.org/10.12659/MSM.928784
V. Todorovova, T. Altschmiedova, M. Vrablik, R. Ceska, Familial Hypercholesterolemia: Real-World Data of 1236 Patients Attending a Czech Lipid Clinic. A Retrospective Analysis of Experience in More than 50 years. Part I: Genetics and Biochemical Parameters. Front Genet 13, 849008 (2022). https://doi.org/10.3389/fgene.2022.849008
T.M. Deneyimi, Evaluation of the Frequency of Familial Hypercholesterolemia: A Single-Center Experience. Turk. J. Endocrinol. Metab. 23, 168–173 (2019). https://doi.org/10.25179/tjem.2019-65855
T. Teramoto, T. Kai, A. Ozaki, B. Crawford, H. Arai, S. Yamashita, Treatment Patterns and Lipid Profile in Patients with Familial Hypercholesterolemia in Japan. J. Atheroscler. Thromb. 25, 580–592 (2018). https://doi.org/10.5551/jat.41483
S. Funabashi, Y. Kataoka, M. Hori, M. Ogura, Y. Nakaoku, K. Nishimura, T. Doi, R. Nishikawa, K. Tsuda, T. Noguchi, M. Harada-Shiba, Substantially Elevated Atherosclerotic Risks in Japanese Severe Familial Hypercholesterolemia Defined by the International Atherosclerosis Society. JACC Asia 1, 245–255 (2021). https://doi.org/10.1016/j.jacasi.2021.07.004
J. Ferrières, M. Farnier, E. Bruckert, A. Vimont, V. Durlach, E. Ferrari, A. Gallo, F. Boccara, D. Ferrières, S. Béliard; French FH Registry group: French REgistry of Familial hypERCHOLesterolemia (REFERCHOL), Burden of cardiovascular disease in a large contemporary cohort of patients with heterozygous familial hypercholesterolemia. Atheroscler 50, 17–24 (2022). https://doi.org/10.1016/j.athplu.2022.08.001
B. Iyen, N. Qureshi, J. Kai, R.K. Akyea, J. Leonardi-Bee, P. Roderick, S.E. Humphries, S. Weng, Risk of cardiovascular disease outcomes in primary care subjects with familial hypercholesterolaemia: A cohort study. Atherosclerosis 287, 8–15 (2019). https://doi.org/10.1016/j.atherosclerosis.2019.05.017
A.M. Galema-Boers, M.J. Lenzen, S.R. Engelkes, E.J. Sijbrands, J.E. Roeters van Lennep, Cardiovascular risk in patients with familial hypercholesterolemia using optimal lipid-lowering therapy. J. Clin. Lipido. 12, 409–416 (2018). https://doi.org/10.1016/j.jacl.2017.12.014
Author contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by E.A., S.D.L., and A.F.G. The first draft of the manuscript was written by E.A., M.M. and A.G. and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
Open access funding provided by Università degli Studi di Roma La Sapienza within the CRUI-CARE Agreement.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
M.M. worked as principal investigator in clinical trials supported by Novo Nordisk. A.G. received consulting fees or payments for lectures, presentations, manuscript writing or educational events from AMGEN, Sanofi and Regeron, Mylan, Ackea Therapeutics, Novartis, MSD, Servier, Ultragenyx. A.G. declares having received support for attending meetings and/or travel by Amryt, Novartis, Ultragenyx and Sanofi, and participated on a data safety monitoring board or advisory board for Sanofi and Ultragenyx.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Acitelli, E., Guedon, A.F., De Liguori, S. et al. Peripheral artery disease: an underdiagnosed condition in familial hypercholesterolemia? A systematic review. Endocrine (2024). https://doi.org/10.1007/s12020-024-03763-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12020-024-03763-x