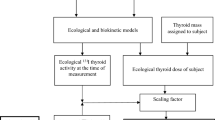
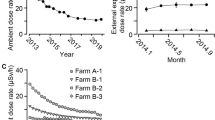
Abstract
Purpose
After the accidents of nuclear power plants at Chernobyl and at Fukushima, huge amounts of radioactive iodine were released into the atmosphere.
Methods
We reviewed data on the health consequences of these accidents with a focus on thyroid consequences.
Results
Among the 2 million children who were living in highly contaminated regions in Belarus, Ukraine and Russia, 7000 cases of thyroid cancer had occurred in 2005. This is the most significant radiation-induced consequence of the Chernobyl accident. The increased incidence of thyroid cancer observed in adult population who lived in these highly contaminated regions is at least in major part related to screening and it is not possible to individualize among these thyroid cancers those that are potentially caused by radiation exposure. For populations who lived outside these regions at the time of the accident, there is no detectable consequence of the radiation exposure on the thyroid gland. Among children who lived nearby the Fukushima power plant in 2011, there is currently no evidence of an increased incidence of thyroid cancer. Ultrasonography screening in these individuals detected a number of thyroid cancers that are probably not related to the accident. Because thyroid cancer is frequent, studies have been carried out to distinguish radiation-induced from their sporadic counterparts, and genomic signatures might be helpful.
Conclusions
The consequences of the Chernobyl accident clearly demonstrate that populations living nearby a nuclear power plant should be protected in case of accident by sheltering, food restrictions and prophylaxis of thyroid irradiation by potassium iodine administration, if the predicted estimated dose to the thyroid gland of children might be >50 mGy. These countermeasures should be applied in priority to children, adolescents and pregnant women; they are safe and effective.

Similar content being viewed by others
References
United Nations Scientific Commission on the Effects of Atomic Radiation (UNSCEAR): UNSCEAR 2013 report—Vol. I https://www.unscear.org/docs/publications/2013/UNSCEAR_2013_Report_Vol.I
R.W. Howell, B.W. Wessels, R. Loevinger, E.E. Watson, W.E. Bolch, A.B. Brill, N.D. Charkes, D.R. Fisher, M.T. Hays, J.S. Robertson, J.A. Siegel, S.R. Thomas, The MIRD perspective 1999. Medical Internal Radiation Dose Committee. J. Nucl. Med. 40, 3S–10S (1999)
J.P. Christodouleas, R.D. Forrest, C.G. Ainsley, Z. Tochner, S.M. Hahn, E. Glatstein, Short-term and long-term health risks of nuclear-power-plant accidents. N. Engl. J. Med. 364, 2334–2341 (2011). https://doi.org/10.1056/NEJMra1103676
United Nations Scientific Commission on the Effects of Atomic Radiation (UNSCEAR) 2008 report—Vol. I: sources. https://www.unscear.org/unscear/en/publications/2008_1.html
United Nations Scientific Commission on the Effects of Atomic Radiation (UNSCEAR) 2013 report—Vol. II. https://www.unscear.org/unscear/en/publications/2013_2.html
WHO 2016: World health statistics 2016: monitoring health for the SDGs, sustainable development goals. (2016). file:///Users/sc157197/Desktop/9789241565264_eng.pdf
United Nations Scientific Commission on the Effects of Atomic Radiation (UNSCEAR) 2008 report—Vol. II: effects. https://www.unscear.org/unscear/en/publications/2008_2.html
V.V. Chumak, Physical dosimetry of chernobyl cleanup workers. Health Physics. 93, 452–461 (2007). https://doi.org/10.1097/01.HP.0000278842.39156.93
United Nations, United Nations: sources and effects of ionizing radiation, UNSCEAR 2008 Report (United Nations, 2011). https://www.unscear.org/docs/publications/2011/UNSCEAR_2011
D.L. Preston, E. Ron, S. Tokuoka, S. Funamoto, N. Nishi, M. Soda, K. Mabuchi, K. Kodama, Solid cancer incidence in atomic bomb survivors: 1958–1998. Radiat. Res. 168, 1–64 (2007). https://doi.org/10.1667/RR0763.1
E. Ostroumova, M. Hatch, A. Brenner, E. Nadyrov, I. Veyalkin, O. Polyanskaya, V. Yauseyenka, S. Polyakov, L. Levin, L. Zablotska, A. Rozhko, K. Mabuchi, Non-thyroid cancer incidence in Belarusian residents exposed to Chernobyl fallout in childhood and adolescence: standardized Incidence Ratio analysis, 1997–2011. Environ. Res. 147, 44–49 (2016). https://doi.org/10.1016/j.envres.2016.01.025
L.N. Astakhova, L.R. Anspaugh, G.W. Beebe, A. Bouville, V.V. Drozdovitch, V. Garber, Y.I. Gavrilin, V.T. Khrouch, A.V. Kuvshinnikov, Y.N. Kuzmenkov, V.P. Minenko, K.V. Moschik, A.S. Nalivko, J. Robbins, E.V. Shemiakina, S. Shinkarev, S.I. Tochitskaya, M.A. Waclawiw, Chernobyl-related thyroid cancer in children of Belarus: a case-control study. Radiat. Res. 150, 349–356 (1998)
M.D. Tronko, G.R. Howe, T.I. Bogdanova, A.C. Bouville, O.V. Epstein, A.B. Brill, I.A. Likhtarev, D.J. Fink, V.V. Markov, E. Greenebaum, V.A. Olijnyk, I.J. Masnyk, V.M. Shpak, R.J. McConnell, V.P. Tereshchenko, J. Robbins, O.V. Zvinchuk, L.B. Zablotska, M. Hatch, N.K. Luckyanov, E. Ron, T.L. Thomas, P.G. Voilleque, G.W. Beebe, A cohort study of thyroid cancer and other thyroid diseases after the chornobyl accident: thyroid cancer in Ukraine detected during first screening. J. Natl. Cancer Inst. 98, 897–903 (2006). https://doi.org/10.1093/jnci/djj244
V.S. Kazakov, E.P. Demidchik, L.N. Astakhova, Thyroid cancer after Chernobyl. Nature 359, 21–21 (1992). https://doi.org/10.1038/359021a0
K. Baverstock, B. Egloff, A. Pinchera, C. Ruchti, D. Williams, Thyroid cancer after Chernobyl. Nature 359, 21–22 (1992). https://doi.org/10.1038/359021b0
E. Ron, Thyroid cancer incidence among people living in areas contaminated by radiation from the Chernobyl accident. Health Phys. 93, 502–511 (2007). https://doi.org/10.1097/01.HP.0000279018.93081.29
M.M. Fuzik, A.Y. Prysyazhnyuk, Y. Shibata, A.Y. Romanenko, Z.P. Fedorenko, N.A. Gudzenko, L.O. Gulak, N.K. Trotsyuk, Y.L. Goroh, O.M. Khukhrianska, O.V. Sumkina, V.A. Saenko, S. Yamashita, Age and gender patterns of thyroid cancer incidence in Ukraine depending on thyroid radiation doses from radioactive iodine exposure after the Chornobyl NPP accident. Probl. Radiac. Med. Radiobiol. 144–155 (2013). PMID: 25191719
P.W. Dickman, L.-E. Holm, G. Lundell, J.D. Boice, P. Hall, Thyroid cancer risk after thyroid examination with 131I: a population-based cohort study in Sweden. Int. J. Cancer 106, 580–587 (2003). https://doi.org/10.1002/ijc.11258
B. Sinnott, E. Ron, A.B. Schneider, Exposing the thyroid to radiation: a review of its current extent, risks, and implications. Endocr. Rev. 31, 756–773 (2010). https://doi.org/10.1210/er.2010-0003
E. Ron, J.H. Lubin, R.E. Shore, K. Mabuchi, B. Modan, L.M. Pottern, A.B. Schneider, M.A. Tucker, J.D. Boice, Thyroid cancer after exposure to external radiation: a pooled analysis of seven studies. 1995. Radiat. Res. 178, AV43–AV60 (2012). https://doi.org/10.1667/rrav05.1
E. Cardis, A. Kesminiene, V. Ivanov, I. Malakhova, Y. Shibata, V. Khrouch, V. Drozdovitch, E. Maceika, I. Zvonova, O. Vlassov, A. Bouville, G. Goulko, M. Hoshi, A. Abrosimov, J. Anoshko, L. Astakhova, S. Chekin, E. Demidchik, R. Galanti, M. Ito, E. Korobova, E. Lushnikov, M. Maksioutov, V. Masyakin, A. Nerovnia, V. Parshin, E. Parshkov, N. Piliptsevich, A. Pinchera, S. Polyakov, N. Shabeka, E. Suonio, V. Tenet, A. Tsyb, S. Yamashita, D. Williams, Risk of thyroid cancer after exposure to 131I in childhood. J. Natl. Cancer Inst. 97, 724–732 (2005). https://doi.org/10.1093/jnci/dji129
L.B. Zablotska, E. Ron, A.V. Rozhko, M. Hatch, O.N. Polyanskaya, A.V. Brenner, J. Lubin, G.N. Romanov, R.J. McConnell, P. O’Kane, V.V. Evseenko, V.V. Drozdovitch, N. Luckyanov, V.F. Minenko, A. Bouville, V.B. Masyakin, Thyroid cancer risk in Belarus among children and adolescents exposed to radioiodine after the Chornobyl accident. Br. J. Cancer 104, 181–187 (2011). https://doi.org/10.1038/sj.bjc.6605967
A.V. Brenner, M.D. Tronko, M. Hatch, T.I. Bogdanova, V.A. Oliynik, J.H. Lubin, L.B. Zablotska, V.P. Tereschenko, R.J. McConnell, G.A. Zamotaeva, P. O’Kane, A.C. Bouville, L.V. Chaykovskaya, E. Greenebaum, I.P. Paster, V.M. Shpak, E. Ron, I-131 dose response for incident thyroid cancers in Ukraine related to the Chornobyl accident. Environ. Health Perspect. 119, 933–939 (2011). https://doi.org/10.1289/ehp.1002674
V.K. Ivanov, V.V. Kashcheev, S.Y. Chekin, M.A. Maksioutov, K.A. Tumanov, O.K. Vlasov, N.V. Shchukina, A.F. Tsyb, Radiation-epidemiological studies of thyroid cancer incidence in Russia after the Chernobyl accident (estimation of radiation risks, 1991-2008 follow-up period). Radiat. Prot. Dosim. 151, 489–499 (2012). https://doi.org/10.1093/rpd/ncs019
E.K. Cahoon, E.A. Nadyrov, O.N. Polyanskaya, V.V. Yauseyenka, I.V. Veyalkin, T.I. Yeudachkova, T.I. Maskvicheva, V.F. Minenko, W. Liu, V. Drozdovitch, K. Mabuchi, M.P. Little, L.B. Zablotska, R.J. McConnell, M. Hatch, K.O. Peters, A.V. Rozhko, A.V. Brenner, Risk of thyroid nodules in residents of Belarus exposed to Chernobyl fallout as children and adolescents. J. Clin. Endocrinol. Metab. 102, 2207–2217 (2017). https://doi.org/10.1210/jc.2016-3842
J.H. Lubin, M.J. Adams, R. Shore, E. Holmberg, A.B. Schneider, M.M. Hawkins, L.L. Robison, P.D. Inskip, M. Lundell, R. Johansson, R.A. Kleinerman, F. de Vathaire, L. Damber, S. Sadetzki, M. Tucker, R. Sakata, L.H.S. Veiga, Thyroid cancer following childhood low-dose radiation exposure: a pooled analysis of nine cohorts. J. Clin. Endocrinol. Metab. 102, 2575–2583 (2017). https://doi.org/10.1210/jc.2016-3529
L.H.S. Veiga, E. Holmberg, H. Anderson, L. Pottern, S. Sadetzki, M.J. Adams, R. Sakata, A.B. Schneider, P. Inskip, P. Bhatti, R. Johansson, G. Neta, R. Shore, F. de Vathaire, L. Damber, R. Kleinerman, M.M. Hawkins, M. Tucker, M. Lundell, J.H. Lubin, Thyroid cancer after childhood exposure to external radiation: an updated pooled analysis of 12 studies. Radiat. Res. 185, 473–484 (2016). https://doi.org/10.1667/RR14213.1
J. Nauman, J. Wolff, Iodide prophylaxis in Poland after the Chernobyl reactor accident: benefits and risks. Am. J. Med. 94, 524–532 (1993). https://doi.org/10.1016/0002-9343(93)90089-8
S. Vaccarella, S. Franceschi, F. Bray, C.P. Wild, M. Plummer, L. Dal Maso, Worldwide thyroid-cancer epidemic? The increasing impact of overdiagnosis. N. Engl. J. Med. 375, 614–617 (2016). https://doi.org/10.1056/NEJMp1604412
M. Li, L.D. Maso, S. Vaccarella, Global trends in thyroid cancer incidence and the impact of overdiagnosis. Lancet Diabetes Endocrinol. 8, 468–470 (2020). https://doi.org/10.1016/S2213-8587(20)30115-7
Fukushima, https://www.unscear.org/unscear/fr/fukushima.html
K. Tanigawa, Y. Hosoi, N. Hirohashi, Y. Iwasaki, K. Kamiya, Loss of life after evacuation: lessons learned from the Fukushima accident. Lancet 379, 889–891 (2012). https://doi.org/10.1016/S0140-6736(12)60384-5
S. Nomura, S. Gilmour, M. Tsubokura, D. Yoneoka, A. Sugimoto, T. Oikawa, M. Kami, K. Shibuya, Mortality risk amongst nursing home residents evacuated after the Fukushima nuclear accident: a retrospective cohort study. PLoS ONE 8, e60192 (2013). https://doi.org/10.1371/journal.pone.0060192
W. Zheng, H. Yokomichi, H. Matsubara, M. Ishikuro, M. Kikuya, T. Isojima, S. Yokoya, T. Tanaka, N. Kato, S. Chida, A. Ono, M. Hosoya, S. Tanaka, S. Kuriyama, S. Kure, Z. Yamagata, Longitudinal changes in body mass index of children affected by the Great East Japan Earthquake. Int J. Obes. 41, 606–612 (2017). https://doi.org/10.1038/ijo.2017.6
M. Orui, Y. Kuroda, S. Yasumura, Suicide rates and mental health measures after the lifting of the evacuation orders following the Fukushima Daiichi Nuclear Power Plant accident: a practical report developed in collaboration with the local municipality. Nihon Koshu Eisei Zasshi. 66, 407–416 (2019). https://doi.org/10.11236/jph.66.8_407
I. Korsakissok, A. Mathieu, D. Didier, Atmospheric dispersion and ground deposition induced by the Fukushima Nuclear Power Plant accident: a local-scale simulation and sensitivity study. Atmos. Environ. 70, 267–279 (2013). https://doi.org/10.1016/j.atmosenv.2013.01.002
M. Tsubokura, M. Murakami, S. Nomura, T. Morita, Y. Nishikawa, C. Leppold, S. Kato, M. Kami, Individual external doses below the lowest reference level of 1 mSv per year five years after the 2011 Fukushima nuclear accident among all children in Soma City. PLoS ONE 12, e0172305 (2017). https://doi.org/10.1371/journal.pone.0172305
S. Nomura, H. Sakamoto, M.K. Sugai, H. Nakamura, K. Maruyama-Sakurai, S. Lee, A. Ishizuka, K. Shibuya, Tracking Japan’s development assistance for health, 2012-2016. Glob. Health 16, 32 (2020). https://doi.org/10.1186/s12992-020-00559-2
M. Miyazaki, A. Ohtsuru, T. Ishikawa, An overview of internal dose estimation using whole-body counters in Fukushima Prefecture. Fukushima J. Med Sci. 60, 95–100 (2014). https://doi.org/10.5387/fms.2014-10
R.S. Hayano, M. Tsubokura, M. Miyazaki, A. Ozaki, Y. Shimada, T. Kambe, T. Nemoto, T. Oikawa, Y. Kanazawa, M. Nihei, Y. Sakuma, H. Shimmura, J. Akiyama, M. Tokiwa, Whole-body counter surveys of over 2700 babies and small children in and around Fukushima Prefecture 33 to 49 months after the Fukushima Daiichi NPP accident. Proc. Jpn Acad. Ser. B Phys. Biol. Sci. 91, 440–446 (2015). https://doi.org/10.2183/pjab.91.440
F. Pacini, T. Vorontsova, E.P. Demidchik, E. Molinaro, L. Agate, C. Romei, E. Shavrova, E.D. Cherstvoy, Y. Ivashkevitch, E. Kuchinskaya, M. Schlumberger, G. Ronga, M. Filesi, A. Pinchera, Post-Chernobyl thyroid carcinoma in Belarus children and adolescents: comparison with naturally occurring thyroid carcinoma in Italy and France. J. Clin. Endocrinol. Metab. 82, 3563–3569 (1997). https://doi.org/10.1210/jcem.82.11.4367
A. Ohtsuru, S. Midorikawa, T. Ohira, S. Suzuki, H. Takahashi, M. Murakami, H. Shimura, T. Matsuzuka, S. Yasumura, S.-I. Suzuki, S. Yokoya, Y. Hashimoto, A. Sakai, H. Ohto, S. Yamashita, K. Tanigawa, K. Kamiya, Incidence of thyroid cancer among children and young adults in Fukushima, Japan, screened with 2 rounds of ultrasonography within 5 years of the 2011 Fukushima Daiichi Nuclear Power Station Accident. JAMA Otolaryngol. Head. Neck Surg. 145, 4–11 (2019). https://doi.org/10.1001/jamaoto.2018.3121
T. Ohira, H. Shimura, F. Hayashi, M. Nagao, S. Yasumura, H. Takahashi, S. Suzuki, T. Matsuzuka, S. Suzuki, M. Iwadate, T. Ishikawa, A. Sakai, S. Suzuki, K.E. Nollet, S. Yokoya, H. Ohto, K. Kamiya; Fukushima Health Management Survey Group, Absorbed radiation doses in the thyroid as estimated by UNSCEAR and subsequent risk of childhood thyroid cancer following the Great East Japan Earthquake. J. Radiat. Res. 61, 243–248 (2020). https://doi.org/10.1093/jrr/rrz104
N. Mitsutake, T. Fukushima, M. Matsuse, T. Rogounovitch, V. Saenko, S. Uchino, M. Ito, K. Suzuki, S. Suzuki, S. Yamashita, BRAF(V600E) mutation is highly prevalent in thyroid carcinomas in the young population in Fukushima: a different oncogenic profile from Chernobyl. Sci. Rep. 5, 16976 (2015). https://doi.org/10.1038/srep16976
S. Midorikawa, S. Suzuki, A. Ohtsuru, After Fukushima: addressing anxiety. Science 352, 666–667 (2016). https://doi.org/10.1126/science.352.6286.666-c
V.S. Perkel, M.H. Gail, J. Lubin, D.Y. Pee, R. Weinstein, E. Shore-Freedman, A.B. Schneider, Radiation-induced thyroid neoplasms: evidence for familial susceptibility factors. J. Clin. Endocrinol. Metab. 66, 1316–1322 (1988). https://doi.org/10.1210/jcem-66-6-1316
M. Zidane, J.-B. Cazier, S. Chevillard, C. Ory, M. Schlumberger, C. Dupuy, J.-F. Deleuze, A. Boland, N. Haddy, F. Lesueur, F. de Vathaire, Genetic susceptibility to radiation-related differentiated thyroid cancers: a systematic review of literature. Endocr. Relat. Cancer 26, R583–R596 (2019). https://doi.org/10.1530/ERC-19-0321
M. Takahashi, V.A. Saenko, T.I. Rogounovitch, T. Kawaguchi, V.M. Drozd, H. Takigawa-Imamura, N.M. Akulevich, C. Ratanajaraya, N. Mitsutake, N. Takamura, L.I. Danilova, M.L. Lushchik, Y.E. Demidchik, S. Heath, R. Yamada, M. Lathrop, F. Matsuda, S. Yamashita, The FOXE1 locus is a major genetic determinant for radiation-related thyroid carcinoma in Chernobyl. Hum. Mol. Genet. 19, 2516–2523 (2010). https://doi.org/10.1093/hmg/ddq123
F. Damiola, G. Byrnes, M. Moissonnier, M. Pertesi, I. Deltour, A. Fillon, F. Le Calvez-Kelm, V. Tenet, S. McKay-Chopin, J.D.McKay, I. Malakhova, V. Masyakin, E. Cardis, F. Lesueur, A. Kesminiene,Contribution of ATM and FOXE1 (TTF2) to risk of papillary thyroid carcinoma in Belarusian children exposed to radiation. Int J. Cancer 134, 1659–1668 (2014). https://doi.org/10.1002/ijc.28483
S. Maillard, F. Damiola, E. Clero, M. Pertesi, N. Robinot, F. Rachedi, J.L. Boissin, J. Sebbag, L. Shan, F. Bost-Bezeaud, P. Petitdidier, F. Doyon, C. Xhaard, C. Rubino, H. Blanche, V. Drozdovitch, F. Lesueur, F. de Vathaire, Common variants at 9q22.33, 14q13.3, and ATM loci, and risk of differentiated thyroid cancer in the French Polynesian population. PLoS ONE 10, e0123700 (2015). https://doi.org/10.1371/journal.pone.0123700
D. Williams, Radiation carcinogenesis: lessons from Chernobyl. Oncogene 27(Suppl 2), S9–S18 (2008). https://doi.org/10.1038/onc.2009.349
J.C. Ricarte-Filho, S. Li, M.E.R. Garcia-Rendueles, C. Montero-Conde, F. Voza, J.A. Knauf, A. Heguy, A. Viale, T. Bogdanova, G.A. Thomas, C.E. Mason, J.A. Fagin, Identification of kinase fusion oncogenes in post-Chernobyl radiation-induced thyroid cancers. J. Clin. Investig. 123, 4935–4944 (2013). https://doi.org/10.1172/JCI69766
R. Ameziane-El-Hassani, M. Boufraqech, O. Lagente-Chevallier, U. Weyemi, M. Talbot, D. Métivier, F. Courtin, J.M. Bidart, M. El Mzibri, M. Schlumberger, C. Dupuy, Role of H2O2 in RET/PTC1 chromosomal rearrangement produced by ionizing radiation in human thyroid cells. Cancer Res. 70, 4123–4132 (2010). https://doi.org/10.1158/CAN-09-4336
B. Pekova, V. Sykorova, S. Dvorakova, E. Vaclavikova, J. Moravcova, R. Katra, J. Astl, P. Vlcek, D. Kodetova, J. Vcelak, B. Bendlova, RET, NTRK, ALK, BRAF and MET fusions in a large cohort of pediatric papillary thyroid carcinomas. Thyroid. (2020). https://doi.org/10.1089/thy.2019.0802
A.S. Alzahrani, M. Alswailem, A.A. Alswailem, H. Al-Hindi, E. Goljan, N. Alsudairy, M. Abouelhoda, Genetic alterations in pediatric thyroid cancer using a comprehensive childhood cancer gene panel. J. Clin. Endocrinol. Metab. (2020). https://doi.org/10.1210/clinem/dgaa389
C. Ory, N. Ugolin, C. Levalois, L. Lacroix, B. Caillou, J.-M. Bidart, M. Schlumberger, I. Diallo, F. de Vathaire, P. Hofman, J. Santini, B. Malfoy, S. Chevillard, Gene expression signature discriminates sporadic from post-radiotherapy-induced thyroid tumors. Endocr.-Relat. Cancer 18, 193–206 (2011). https://doi.org/10.1677/ERC-10-0205
N. Ugolin, C. Ory, E. Lefevre, N. Benhabiles, P. Hofman, M. Schlumberger, S. Chevillard, Strategy to find molecular signatures in a small series of rare cancers: validation for radiation-induced breast and thyroid tumors. PLoS ONE 6, e23581 (2011). https://doi.org/10.1371/journal.pone.0023581
V. Detours, L. Delys, F. Libert, D. Weiss Solís, T. Bogdanova, J.E. Dumont, B. Franc, G. Thomas, C. Maenhaut, Genome-wide gene expression profiling suggests distinct radiation susceptibilities in sporadic and post-Chernobyl papillary thyroid cancers. Br. J. Cancer 97, 818–825 (2007). https://doi.org/10.1038/sj.bjc.6603938
M. Port, C. Boltze, Y. Wang, B. Röper, V. Meineke, M. Abend, A radiation-induced gene signature distinguishes post-Chernobyl from sporadic papillary thyroid cancers. Radiat. Res. 168, 639–649 (2007). https://doi.org/10.1667/RR0968.1
L. Stein, J. Rothschild, J. Luce, J.K. Cowell, G. Thomas, T.I. Bogdanova, M.D. Tronko, L. Hawthorn, Copy number and gene expression alterations in radiation-induced papillary thyroid carcinoma from chernobyl pediatric patients. Thyroid. Off. J. Am. Thyroid. Assoc. 20, 475–487 (2010). https://doi.org/10.1089/thy.2009.0008
C. Ory, N. Ugolin, P. Hofman, M. Schlumberger, I.A. Likhtarev, S. Chevillard, Comparison of transcriptomic signature of post-Chernobyl and postradiotherapy thyroid tumors. Thyroid. Off. J. Am. Thyroid. Assoc. 23, 1390–1400 (2013). https://doi.org/10.1089/thy.2012.0318
M. Abend, R.M. Pfeiffer, C. Ruf, M. Hatch, T.I. Bogdanova, M.D. Tronko, J. Hartmann, V. Meineke, K. Mabuchi, A.V. Brenner, Iodine-131 dose-dependent gene expression: alterations in both normal and tumour thyroid tissues of post-Chernobyl thyroid cancers. Br. J. Cancer 109, 2286–2294 (2013). https://doi.org/10.1038/bjc.2013.574
C. Sermage-Faure, D. Laurier, S. Goujon-Bellec, M. Chartier, A. Guyot-Goubin, J. Rudant, D. Hémon, J. Clavel, Childhood leukemia around French nuclear power plants-the Geocap study, 2002-2007. Int. J. Cancer 131, E769–E780 (2012). https://doi.org/10.1002/ijc.27425
P.B. Zanzonico, D.V. Becker, Effects of time of administration and dietary iodine levels on potassium iodide (KI) blockade of thyroid irradiation by 131I from radioactive fallout. Health Phys. 78, 660–667 (2000). https://doi.org/10.1097/00004032-200006000-00008
Journal Officiel de la République Française (JORF), n°136 du 14 juin 2006 page 8946 texte n° 2 LOI n° 2006-686 du 13 juin 2006 relative à la transparence et à la sécurité
B. Le Guen, L. Stricker, M. Schlumberger, Distributing KI pills to minimize thyroid radiation exposure in case of a nuclear accident in France. Nat. Clin. Pract. Endocrinol. Metab. 3, 611–611 (2007). https://doi.org/10.1038/ncpendmet0593
L. Vydro, C.M. Kitahara, J.H. Lubin, A.B. Schneider, D.V. Mihailescu, Among individuals irradiated for benign conditions in childhood, developing thyroid cancer does not affect all-cause survival. Thyroid. Off. J. Am. Thyroid. Assoc. 30, 389–395 (2020). https://doi.org/10.1089/thy.2019.0439
B. Le Guen, L. Stricker, M. Schlumberger, Distributing KI pills to minimize thyroid radiation exposure in case of a nuclear accident in France. Nat. Clin. Pract. Endocrinol. Metab. 3, 611–611 (2007). https://doi.org/10.1038/ncpendmet0593
S. Vaccarella, L. Dal Maso, M. Laversanne, F. Bray, M. Plummer, S. Franceschi, The impact of diagnostic changes on the rise in thyroid cancer incidence: a population-based study in selected high-resource countries. Thyroid. Off. J. Am. Thyroid. Assoc. 25, 1127–1136 (2015). https://doi.org/10.1089/thy.2015.0116
B.R. Haugen, E.K. Alexander, K.C. Bible, G.M. Doherty, S.J. Mandel, Y.E. Nikiforov, F. Pacini, G.W. Randolph, A.M. Sawka, M. Schlumberger, K.G. Schuff, S.I. Sherman, J.A. Sosa, D.L. Steward, R.M. Tuttle, L. Wartofsky, 2015 American Thyroid Association Management Guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association Guidelines Task Force on thyroid nodules and differentiated thyroid cancer. Thyroid 26, 1–133 (2016). https://doi.org/10.1089/thy.2015.0020
S.C. Clement, L.C.M. Kremer, F.A. Verburg, J.H. Simmons, M. Goldfarb, R.P. Peeters, E.K. Alexander, E. Bardi, E. Brignardello, L.S. Constine, C.A. Dinauer, V.M. Drozd, F. Felicetti, E. Frey, A. Heinzel, M.M. van den Heuvel-Eibrink, S.A. Huang, T.P. Links, K. Lorenz, R.L. Mulder, S.J. Neggers, E.J.M. Nieveen van Dijkum, K.C. Oeffinger, R.R. van Rijn, S.A. Rivkees, C.M. Ronckers, A.B. Schneider, R. Skinner, J.D. Wasserman, T. Wynn, M.M. Hudson, P.C. Nathan, H.M. van Santen, Balancing the benefits and harms of thyroid cancer surveillance in survivors of Childhood, adolescent and young adult cancer: recommendations from the international Late Effects of Childhood Cancer Guideline Harmonization Group in collaboration with the PanCareSurFup Consortium. Cancer Treat. Rev. 63, 28–39 (2018). https://doi.org/10.1016/j.ctrv.2017.11.005
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
B.L.G. is employee at Electricité de France. F.D.V., C.O., and S.C. received research supports from Electricité de France. M.S. is consultant for Electricité de France.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ory, C., Leboulleux, S., Salvatore, D. et al. Consequences of atmospheric contamination by radioiodine: the Chernobyl and Fukushima accidents. Endocrine 71, 298–309 (2021). https://doi.org/10.1007/s12020-020-02498-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-020-02498-9