Abstract
Purposes
We aimed to assess the effects of recombinant human growth hormone (rhGH) replacement therapy on metabolic changes by synthesizing data from clinical trials involving children with idiopathic growth hormone deficiency (IGHD).
Methods
Two investigators independently completed literature search, quality assessment, and data extraction. Effect-size estimates are expressed as weighted mean difference (WMD) with 95% confidence interval (CI).
Results
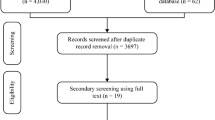
A total of 16 clinical trials involving 1319 children were eligible for analysis. Overall analyses showed that total cholesterol was significantly decreased after rhGH replacement therapy (WMD: −0.20 mmol/l; 95% CI: −0.30 to −0.10; p < 0.001), and high-density lipoprotein was significantly increased (WMD: 0.29 mmol/l; 95% CI: 0.24 to 0.33; p < 0.001). Marginal increase was noted for low-density lipoprotein (WMD: −0.22 mmol/l; 95% CI: −0.47 to 0.22; p = 0.092). Subsidiary and meta-regression analyses revealed that length of intervention and sample size were possible causes of heterogeneity. There was a low probability of publication bias.
Conclusions
Our findings indicate an obviously favorable role of rhGH replacement therapy in lipid metabolism in children with IGHD, and this role might be dependent on length of intervention.





Similar content being viewed by others
References
X.L. Bao, Y.F. Shi, Y.C. Du, R. Liu, J.Y. Deng, S.M. Gao, Prevalence of growth hormone deficiency of children in Beijing. Chin. Med. J. 105(5), 401–405 (1992)
A. Chinoy, P.G. Murray, Diagnosis of growth hormone deficiency in the paediatric and transitional age. Best Pract. Res. Clin. Endocrinol. Metab. 30(6), 737–747 (2016). https://doi.org/10.1016/j.beem.2016.11.002
R.C. Lindsey, S. Mohan, Skeletal effects of growth hormone and insulin-like growth factor-I therapy. Mol. Cell Endocrinol. 432, 44–55 (2016). https://doi.org/10.1016/j.mce.2015.09.017
D. Capalbo, A. Esposito, R. Di Mase, F. Barbieri, G. Parenti, P. Vajro, C. Pignata, M. Salerno, Update on early cardiovascular and metabolic risk factors in children and adolescents affected with growth hormone deficiency. Minerva Endocrinol. 37(4), 379–389 (2012)
D. Capalbo, F. Barbieri, N. Improda, F. Giallauria, E. Di Pietro, A. Rapacciuolo, R. Di Mase, C. Vigorito, M. Salerno, Growth hormone improves cardiopulmonary capacity and body composition in children with growth hormone deficiency. J. Clin. Endocrinol. Metab. 102(11), 4080–4088 (2017). https://doi.org/10.1210/jc.2017-00871
J.H. Quitmann, A.C. Rohenkohl, U. Kammerer, C. Schofl, M. Bullinger, H.G. Dorr, [Quality of life of young adults after a growth hormone therapy with childhood onset]. Dtsch. Med. Wochenschr. 139(46), 2335–2338 (2014). https://doi.org/10.1055/s-0034-1387314
R. Lanes, Cardiovascular risk in growth hormone deficiency: beneficial effects of growth hormone replacement therapy. Endocrinol. Metab. Clin. North. Am. 45(2), 405–418 (2016). https://doi.org/10.1016/j.ecl.2016.01.005
G. Saggese, M.B. Ranke, P. Saenger, R.G. Rosenfeld, T. Tanaka, J.L. Chaussain, M.O. Savage, Diagnosis and treatment of growth hormone deficiency in children and adolescents: towards a consensus. Ten years after the Availability of Recombinant Human Growth Hormone Workshop held in Pisa, Italy, 27-28 March 1998. Horm. Res. 50(6), 320–340 (1998). https://doi.org/10.1159/000023298
C. De Leonibus, S. De Marco, A. Stevens, P. Clayton, F. Chiarelli, A. Mohn, Growth hormone deficiency in prepubertal children: predictive markers of cardiovascular disease. Horm. Res. Paediatr. 85(6), 363–371 (2016). https://doi.org/10.1159/000444143
F. Prodam, P. Marzullo, G. Aimaretti, Growth hormone deficiency in children. Best Pract. Res. Clin. Endocrinol. Metab. 30(6), 677–678 (2016). https://doi.org/10.1016/j.beem.2016.12.001
S.R. Daniels, F.R. Greer; Committee on Nutrition, Lipid screening and cardiovascular health in childhood. Pediatrics 122(1), 198–208 (2008). https://doi.org/10.1542/peds.2008-1349
A. Pires, C. Sena, R. Seica, Dyslipidemia and cardiovascular changes in children. Curr. Opin. Cardiol. 31(1), 95–100 (2016). https://doi.org/10.1097/HCO.0000000000000249
J. Rothermel, C. Knop, N. Lass, C. Toschke, R. Wunsch, T. Reinehr, Carotid intima-media thickness in children treated with growth hormone: a follow-up study over three years. Horm. Res. Paediatr. 87(2), 73–80 (2017). https://doi.org/10.1159/000450643
M. Chen, D. Gan, Y. Luo, S. Rampersad, L. Xu, S. Yang, N. Li, H. Li, Effect of recombinant human growth hormone therapy on blood lipid and carotid intima-media thickness in children with growth hormone deficiency. Pediatr. Res. 83(5), 954–960 (2018). https://doi.org/10.1038/pr.2017.271
M. Crowther, W. Lim, M.A. Crowther, Systematic review and meta-analysis methodology. Blood 116(17), 3140–3146 (2010). https://doi.org/10.1182/blood-2010-05-280883
K. Slim, E. Nini, D. Forestier, F. Kwiatkowski, Y. Panis, J. Chipponi, Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J. Surg. 73(9), 712–716 (2003)
S. Duval, R. Tweedie, Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 56(2), 455–463 (2000). https://doi.org/10.1111/j.0006-341x.2000.00455.x
W. Lu, S. Shen, X. Luo, C. Gong, X. Gu, Y. Li, M. Du, R. Jin, Q. Zhou, W. Wang, Comparative evaluation of short-term biomarker response to treatment for growth hormone deficiency in Chinese children with growth hormone deficiency born small for or appropriate for gestational age: a randomized phase IV open-label study. Ther. Adv. Endocrinol. Metab. 4(2), 41–49 (2013). https://doi.org/10.1177/2042018813484051
A. Ciresi, F. Ciccio, S. Radellini, V. Guarnotta, A.M. Calcaterra, C. Giordano, More favorable metabolic impact of three-times-weekly versus daily growth hormone treatment in naive GH-deficient children. Int. J. Endocrinol. 2017, 8469680 (2017). https://doi.org/10.1155/2017/8469680
X. Luo, L. Hou, L. Liang, G. Dong, S. Shen, Z. Zhao, C.X. Gong, Y. Li, M.L. Du, Z. Su, H. Du, C. Yan, Long-acting PEGylated recombinant human growth hormone (Jintrolong) for children with growth hormone deficiency: phase II and phase III multicenter, randomized studies. Eur. J. Endocrinol. 177(2), 195–205 (2017). https://doi.org/10.1530/EJE-16-0905
C. Foster, A. Burton, J. Scholl, M.L. Scott, V. Gunter, K. McCormick, Lipid patterns in treated growth hormone deficient children vs. short stature controls. J. Pediatr. Endocrinol. Metab. 27(9–10), 909–914 (2014). https://doi.org/10.1515/jpem-2013-0488
E.O. Reiter, K.M. Attie, T. Moshang Jr., B.L. Silverman, S.F. Kemp, R.B. Neuwirth, K.M. Ford, P. Saenger, Genentech IAICSG, A multicenter study of the efficacy and safety of sustained release GH in the treatment of naive pediatric patients with GH deficiency. J. Clin. Endocrinol. Metab. 86(10), 4700–4706 (2001). https://doi.org/10.1210/jcem.86.10.7932
N. Mauras, K.M. Attie, E.O. Reiter, P. Saenger, J. Baptista, High dose recombinant human growth hormone (GH) treatment of GH-deficient patients in puberty increases near-final height: a randomized, multicenter trial. Genentech, Inc., Cooperative Study Group. J. Clin. Endocrinol. Metab. 85(10), 3653–3660 (2000). https://doi.org/10.1210/jcem.85.10.6906
P. Cohen, J. Germak, A.D. Rogol, W. Weng, A.M. Kappelgaard, R.G. Rosenfeld; American Norditropin Study Group, Variable degree of growth hormone (GH) and insulin-like growth factor (IGF) sensitivity in children with idiopathic short stature compared with GH-deficient patients: evidence from an IGF-based dosing study of short children. J. Clin. Endocrinol. Metab. 95(5), 2089–2098 (2010). https://doi.org/10.1210/jc.2009-2139
Y. Xue, Y. Gao, S. Wang, P. Wang, An examination of the effects of different doses of recombinant human growth hormone on children with growth hormone deficiency. Exp. Ther. Med. 11(5), 1647–1652 (2016). https://doi.org/10.3892/etm.2016.3091
A. Ciresi, S. Radellini, V. Guarnotta, M.G. Mineo, C. Giordano, The metabolic outcomes of growth hormone treatment in children are gender specific. Endocr. Connect. 7(7), 879–887 (2018). https://doi.org/10.1530/EC-18-0135
F. Peter, M. Bidlingmaier, C. Savoy, H.J. Ji, P.H. Saenger, Three-year efficacy and safety of LB03002, a once-weekly sustained-release growth hormone (GH) preparation, in prepubertal children with GH deficiency (GHD). J. Clin. Endocrinol. Metab. 97(2), 400–407 (2012). https://doi.org/10.1210/jc.2011-2234
G. Radetti, F. Buzi, C. Paganini, C. Martelli, S. Adami, A four year dose-response study of recombinant human growth hormone treatment of growth hormone deficient children: effects on growth, bone growth and bone mineralization. Eur. J. Endocrinol. 142(1), 42–46 (2000). https://doi.org/10.1530/eje.0.1420042
W.V. Moore, H.J. Nguyen, G.B. Kletter, B.S. Miller, D. Rogers, D. Ng, J.A. Moore, E. Humphriss, J.L. Cleland, G.M. Bright, A. Randomized Safety and Efficacy Study of Somavaratan (VRS-317), a long-acting rhGH, in pediatric growth hormone deficiency. J. Clin. Endocrinol. Metab. 101(3), 1091–1097 (2016). https://doi.org/10.1210/jc.2015-3279
M. Rudling, G. Norstedt, H. Olivecrona, E. Reihner, J.A. Gustafsson, B. Angelin, Importance of growth hormone for the induction of hepatic low density lipoprotein receptors. Proc. Natl Acad. Sci. USA 89(15), 6983–6987 (1992). https://doi.org/10.1073/pnas.89.15.6983
H. Olivecrona, S. Ericsson, B. Angelin, Growth hormone treatment does not alter biliary lipid metabolism in healthy adult men. J. Clin. Endocrinol. Metab. 80(4), 1113–1117 (1995). https://doi.org/10.1210/jcem.80.4.7714078
S. Eden, O. Wiklund, J. Oscarsson, T. Rosen, B.A. Bengtsson, Growth hormone treatment of growth hormone-deficient adults results in a marked increase in Lp(a) and HDL cholesterol concentrations. Arterioscler. Thromb. 13(2), 296–301 (1993). https://doi.org/10.1161/01.atv.13.2.296
S. Lind, M. Rudling, S. Ericsson, H. Olivecrona, M. Eriksson, B. Borgstrom, G. Eggertsen, L. Berglund, B. Angelin, Growth hormone induces low-density lipoprotein clearance but not bile acid synthesis in humans. Arterioscler. Thromb. Vasc. Biol. 24(2), 349–356 (2004). https://doi.org/10.1161/01.ATV.0000110657.67317.90
A.M. Mahmoud, R.J. Hernandez Bautista, M.A. Sandhu, O.E. Hussein, Beneficial effects of citrus flavonoids on cardiovascular and metabolic health. Oxid. Med. Cell. Longev. 2019, 5484138 (2019). https://doi.org/10.1155/2019/5484138
O. Guardamagna, F. Abello, G. Anfossi, M. Pirro, Lipoprotein(a) and family history of cardiovascular disease in children with familial dyslipidemias. J. Pediatr. 159(2), 314–319 (2011). https://doi.org/10.1016/j.jpeds.2011.01.038
Z. Quijada, M. Paoli, Y. Zerpa, N. Camacho, R. Cichetti, V. Villarroel, G. Arata-Bellabarba, R. Lanes, The triglyceride/HDL-cholesterol ratio as a marker of cardiovascular risk in obese children; association with traditional and emergent risk factors. Pediatr. Diabetes 9(5), 464–471 (2008). https://doi.org/10.1111/j.1399-5448.2008.00406.x
D. Capalbo, A. Lo Vecchio, V. Farina, L. Spinelli, A. Palladino, C. Tiano, T. Lettiero, G. Lombardi, A. Colao, M. Salerno, Subtle alterations of cardiac performance in children with growth hormone deficiency: results of a two-year prospective, case-control study. J. Clin. Endocrinol. Metab. 94(9), 3347–3355 (2009). https://doi.org/10.1210/jc.2008-2639
E. Gomez-Guzman, M.D. Canete, R. Valle-Martos, R. Canete, M. Valle, L. Jimenez-Reina, J. Caballero-Villarraso, Short-term evaluation of left ventricular mass and function in children with growth hormone deficiency after replacement treatment. Front. Pediatr. 6, 174 (2018). https://doi.org/10.3389/fped.2018.00174
C. Azzarito, L. Boiardi, M. Zini, A. Agosti, M. Biacchessi, R. Biagi, I. Portioli, Short and long-term effects of growth hormone treatment on lipid, lipoprotein, and apolipoprotein levels in short normal children. Horm. Metab. Res. 26(9), 432–435 (1994). https://doi.org/10.1055/s-2007-1001724
K.A. Metwalley, H.S. Farghaly, H.A. Abd El-Hafeez, Evaluation of left ventricular mass and function, lipid profile, and insulin resistance in Egyptian children with growth hormone deficiency: a single-center prospective case-control study. Indian J. Endocrinol. Metab. 17(5), 876–882 (2013). https://doi.org/10.4103/2230-8210.117234
C.J. Child, A.G. Zimmermann, R.S. Scott, G.B. Cutler Jr., T. Battelino, W.F. Blum; GeNe SISIAB, Prevalence and incidence of diabetes mellitus in GH-treated children and adolescents: analysis from the GeNeSIS observational research program. J. Clin. Endocrinol. Metab. 96(6), E1025–E1034 (2011). https://doi.org/10.1210/jc.2010-3023
F. Baronio, L. Mazzanti, Y. Girtler, F. Tamburrino, A. Fazzi, F. Lupi, S. Longhi, G. Radetti, The influence of growth hormone treatment on glucose homeostasis in growthhormone-deficient children: a six-year follow-up study. Horm. Res. Paediatr. 86(3), 196–200 (2016). https://doi.org/10.1159/000448841
Author information
Authors and Affiliations
Contributions
Conceived and designed the experiments: Y.Y., Z.Z., and W.N. Performed the experiments: Y.Y., B.Z., and W.N. Analyzed the data: Y.Y. and W.N. Contributed materials/analysis tools: Y.Y., B.Z., S.L., and Z.Z. Wrote the paper: Y.Y. and W.N. All authors read and approved the final manuscript prior to submission.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Ethical approval is waived for the present study because this is a meta-analysis based on the results of individual studies that had been reviewed and approved by localized ethics committees.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Yuan, Y., Zhou, B., Liu, S. et al. Meta-analysis of metabolic changes in children with idiopathic growth hormone deficiency after recombinant human growth hormone replacement therapy. Endocrine 71, 35–46 (2021). https://doi.org/10.1007/s12020-020-02435-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-020-02435-w