Abstract
Purpose
This essay aims to propose suggestions on what we can learn from previous investigations to conduct further studies on the potential mechanisms underlying the effect of diabetes mellitus on COVID-19.
Methods
We reviewed some literature on diabetes and other types of coronavirus infection such as Middle East respiratory syndrome (MERS) and severe acute respiratory syndrome (SARS) and made some summaries and comparisons.
Results
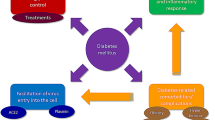
Diabetes affect the occurrence and progression of COVID-19.
Conclusions
In-depth and comprehensive exploration of the mechanism of diabetes affecting COVID-19 should be carried out.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
In recent months, an outbreak of Coronavirus disease 2019 (COVID-19) infected by a new coronavirus, severe acute respiratory syndrome (SARS-CoV-2), has been confirmed and spread in more than 200 countries, areas or territories around the world [1, 2]. COVID-19 has inflicted more than four million people worldwide with a mortality rate of 6.8% according to the situation report of WHO on May 16, 2020 [3]. In addition to effectively preventing and controlling the disease and ending the pandemic as soon as possible, it is very important to optimize the treatment strategy and improve the prognosis of patients.
Diabetes is a chronic noncommunicable disease, more than 460 million people have it around the world. Therefore, a significant number of COVID-19 patients have diabetes. It is also one of the important risk factors of the progression and poor prognosis of COVID-19 [4]. According to three different retrospective studies, among the patients with COVID-19, 17–19% of them have diabetes [5,6,7]. COVID-19 patients with diabetes have a crude mortality rate of about 7.3%, much higher than that of COVID-19 patients without diabetes (0.9%) [8]. The percentage of diabetic patients in intensive care unit (ICU) is about 22.2%; also higher than those without diabetes (5.9%) [9]. Four studies reported that 21–35% patients had diabetes among the severe cases of death [5,6,7, 10]. Qiao et al. find that the average glycosylated hemoglobin of the nonsurvivors was 9.9%, and the average random blood glucose of the nonsurvivors is 13.5 mmol/l, which is significantly higher than that of the survivors [7].
Given the situation, basic and clinical researches on COVID-19 combined with diabetes should be carried out to find intervention strategies for patients, and reduce the mortality.
Recent studies suggest angiotensin-converting enzyme 2 (ACE2), the surface receptor for SARS coronavirus (SARS-CoV), may take part in the mechanism [11]. ACE2 is also expressed in pancreas, SARS-CoV can inutilize ACE2 to bind and damage islets [12]. Scientists have also recently suggested that there is a bidirectional relationship between Covid-19 and diabetes. Although there are data suggesting that SARS-CoV may be a factor leading to new-onset diabetes, there is no direct evidence that SARS-CoV infection will induce diabetes. Executing animal trails to explore the pathogenesis will be important. However, endocrinologist Paul Stewart pointed out in his review that the adverse effects of diabetes on COVID-19 may not be directly due to diabetes, but may be due to other risk factors such as age [13]. So far, to the best of our knowledge, the mechanism of the adverse effects of diabetes on COVID-19 disease remains unclear. It leaves us a very important and valuable area of research. The impact of diabetes on other infectious diseases caused by coronavirus has been studied, such as Middle East respiratory syndrome (MERS) [14] and severe acute respiratory syndrome (SARS) [15]. Previous studies on diabetes and other coronavirus infections may shed light on the research regarding COVID-19 disease.
MERS is a viral respiratory disease caused by MERS-CoV [16]. It has been reported that 88% (n = 15) of the 17 patients with diabetes and MERS required ICU treatment or death, which is much higher than that of MERS patients without diabetes (39%) [17]. Several other studies have reported that MERS patients with diabetes have a critical or fatal MERS risk ratio of 2.47–7.2 [18, 19]. According to epidemiological data, although the mortality rate of MERS is much higher than that of COVID-19, it still has many clinical features similar to COVID-19. Therefore, the research on the mechanism of the occurrence and development of MERS can be used for reference of COVID-19.
Through MERS susceptible animal models, a systematic study on the mechanism of diabetes on MERS was conducted [20]. Its research strategy is worth learning and can give some enlightenment. Compared with the diabetic group, the high-fat feeding group has metabolic abnormalities such as obesity and insulin resistance but had little effect on the severity of MERS suggesting that poor blood glucose control may be the source of a series of immune disorders and disease deterioration after MERS-CoV infection. The immune imbalance is an essential mechanism by which diabetes exacerbates the condition of MERS. Following infection with MERS-CoV, diabetes affects the number and type ratio of immune cells, chemokines, and cytokines in the lungs, resulting in delayed immune response and slow repair of inflammatory damage, thus making lung damage persist for a long time. It will also be an important part of the mechanism study of the effect of diabetes on COVID-19 that fully revealing the specific manifestations and occurrence processes of immune imbalances. SARS caused by a novel coronavirus also posed a severe threat to international health in November 2002. In a retrospective analysis [21], the presence of diabetes mellitus increased the mortality risk. Diabetes and hyperglycemia were independent predictors for death and morbidity in SARS patients after controlling for age and gender. In addition to the lungs, ACE2 is also expressed in the kidney, heart, gastrointestinal tract, brain, and pancreas. SARS-CoV may damage islets and cause acute insulin dependent diabetes mellitus through ACE2 [12]. Chan et al. [21] found immunostaining for ACE2 protein was strong in the pancreatic islets but very weak in the exocrine tissues. In the MERS study, due to the wide expression of ACE2 multiple organsdamage are common and are closely related to poor prognosis [20]. The host cell binding receptor of COVID-19 is ACE2 [22]. Multiple organsdamage are common in COVID-19 and are closely related to poor prognosis, so the study on the expression level and activity of SARS-CoV-2 in extrapulmonary tissues in diabetic and nondiabetic patients cannot be ignored. It should be studied whether SARS-CoV-2 can also aggravate diabetes through this approach, and then superimpose the COVID-19 disease that adverse affects diabetic patients.
Although clinical cases can also be used to understand the effects of diabetes on immune imbalance and extrapulmonary injury in COVID-19 patients, a detailed and in-depth mechanism cannot be discussed without a suitable animal model. The establishment of a mouse model is essential to explore the specific mechanism of diabetes affecting COVID-19.
Since COVID-19, SARS, and MERS have similar etiological categories and clinical characteristics, they are, after all, different kinds of viruses. The host cells bind to different receptors, and the mortalities varies greatly. Therefore, in addition to the enlightenment provided by the MERS study, other possible mechanism research pointcut must also be considered.
For example, (1) Previous studies have found that diabetes can significantly reduce the body’s ability to control the replication of Mycobacterium tuberculosis and Pseudomonas [23]. Therefore, it can be explored whether different hyperglycemia states affect the 2019-CoV replication state in the body. (2) Dendritic cells (DC) are an important factor linking innate immunity and obtaining immunity. It has been reported that diabetes can promote the development of tuberculosis by significantly reducing the number and role of DC subgroups [24]; therefore, DC can also be used as one of the research targets of diabetes combined with COVID-19.
Many diabetic patients also use a variety of other therapeutic drugs, and the impact of these commonly used drugs on COVID-19 is also worth studying. For example, angiotensinase inhibitor (ACEI) and angiotensin II receptor antagonist (ARB) drugs are the recommended drugs for people with diabetes and hypertension or kidney disease. Their influence on the expression and activity of ACE2 in lung tissue is still inconclusive. A meta-analysis on the relationship between ACEI/ARB and risk of pneumonia suggests that ACEI may reduce the risk of pneumonia in individuals (especially Asians) and is related to the reduced pneumonia-related mortality [25]. Another example: In recent years, in vivo and in vitro studies have suggested that metformin and statins may have immunomodulatory and anti-inflammatory effects [26,27,28]; after insulin intervention in diabetic mice, the expression of ACE2 protein in the lung tissue can be significantly reduced; while no significant change inactivity [29]. Besides, Faraaz found that GLP-1, which is one of the novel anti-diabetic drugs, could promote the maintenance of euglycemia and modulate the host inflammatory response in the care of critically ill patients with sepsis [30]. It is necessary to explore the effects and mechanisms of these drugs on COVID-19 disease in diabetic and nondiabetic patients.
In summary, clinical epidemiology has found that diabetes affect the occurrence and progression of COVID-19. An in-depth and comprehensive exploration of the mechanism of diabetes affecting COVID-19 should be carried out. It will contribute to better understanding of COVID-19, guide the clinical management of patients with diabetes and COVID-19, and reduce the severe rate and mortality caused by COVID-19. We call on endocrinologists and epidemiologists to invest more in the specific mechanisms underlying COVID-19 in diabetes mellitus and to draw more useful lessons from basic research on other types of coronavirus. At the same time, health professionals themselves run a risk of COVID-19 virus infection. Safety training, adequate personal protective equipment, regular physical examination and biochemical test, as well as the establishment of an emergency plan and the provision of psychological support for health care workers are of vital importance. Their safety is conducive to the stability of medical teams and the quality of medical services.
References
Q. Li, X. Guan, P. Wu et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med 382(13), 1199–1207 (2020)
H. Tu, S. Tu, S. Gao et al. The epidemiological and clinical features of COVID-19 and lessons from this global infectious public health event. J Infect. 18, S0163–S4453 (2020)
Coronavirus disease 2019 (COVID-19) situation report. Coronavirus disease (COVID-19)/Diseases/Emergencies/Home/WHO. https://www.who.int/emergencies/diseases/novel-coronavirus-2019
W. Guo, M. Li, Y. Dong et al. Diabetes is a risk factor for the progression and prognosis of COVID-19. Diabetes Metab Res Rev. e3319 (2020). https://doi.org/10.1002/dmrr.3319. [Online ahead of print]
T. Chen, D. Wu, H. Chen et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 368, m1295 (2020)
F. Zhou, T. Yu, R. Du, Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 395(10229), 1054–1062 (2020)
Q. Shi, X. Zhang, F. Jiang, Diabetic patients with COVID-19, characteristics and outcome—a two-centre, retrospective, case control study. SSRN Electronic Journal (2020)
Y.P. Zhang, The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China.chin. J Epidemiol 41(2), 145–151 (2020)
D. Wang, B. Hu, C. Hu et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 323(11), 1061–1069 (2020)
X. Yang, Y. Yu, J. Xu et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet 8(5), 475–481 (2020)
M. Letko, A. Marzi, V. Munster, Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage B betacoronaviruses. Nat Microbiol. 5(4), 562–569 (2020)
J.-K. Yang, S.-S. Lin, X.-J. Ji, et al., Binding of SARS coronavirus to its receptor damages islets and causes acute diabetes. Acta Diabetol. 47(3), 193–199 (2010).
S. Gordon, Why is coronavirus a bigger worry for people with diabetes? HealthDay News. (2020). https://www.usnews.com/news/health-news/articles/2020-04-13/why-is-coronavirus-a-bigger-worry-for-people-with-diabetes
F.Y. Alqahtani, F.S. Aleanizy, R. Ali El Hadi Mohamed et al. Prevalence of comorbidities in cases of Middle East respiratory syndrome coronavirus: a retrospective study. Epidemiol Infect. 147, 1–5 (2018)
C.M. Booth, L.M. Matukas, G.A. Tomlinson et al. Clinical features and short-term outcomes of 144 patients with SARS in the greater Toronto area. JAMA. 289(21), 2801–2809 (2003)
M. Cotten, T.T. Lam, S.J. Watson et al. Full-genome deep sequencing and phylogenetic analysis of novel human betacoronavirus. Emerg Infect Dis 19(5), 736–742 (2013)
M.A. Garbati, S.F. Fagbo, V.J. Fang et al. A comparative study of clinical presentation and risk factors for adverse outcome in patients hospitalised with acute respiratory disease due to MERS coronavirus or other causes. PLoS ONE 11(11), e0165978 (2016)
J.A. Al-Tawfiq, K. Hinedi, J. Ghandour et al. Middle East respiratory syndrome coronavirus: a case-control study of hospitalized patients. Clin Infect Dis 59(2), 160–165 (2014)
F.Y. Alqahtani, F.S. Aleanizy, R. Ali El Hadi Mohamed et al. Prevalence of comorbidities in cases of Middle East respiratory syndrome coronavirus: a retrospective study. Epidemiol Infect 147, e35 (2019)
K.A. Kulcsar, C.M. Coleman, S.E. Beck et al. Comorbid diabetes results in immune dysregulation and enhanced disease severity following MERS-CoV infection. JCI Insight 4(20), e131774 (2019)
J.W.M. Chan, C.K. Ng, Y.H. Chan et al. Short term outcome and risk factors for adverse clinical outcomes in adults with severe acute respiratory syndrome (SARS). Thorax 58, 686–689 (2003)
F. Wu, S. Zhao, B. Yu et al. A new coronavirus associated with human respiratory disease in China. Nature 579(7798), 265–269 (2020)
K. Hodgson, J. Morris, T. Bridson et al. Immunological mechanisms contributing to the double burden of diabetes and intracellular bacterial infections. Immunology 144(2), 171–185 (2015)
P. Kumar Nathella, S. Babu, Influence of diabetes mellitus on immunity to human tuberculosis. Immunology 152(1), 13–24 (2017)
D. Caldeira, J. Alarcão, A. Vaz-Carneiro et al. Risk of pneumonia associated with use of angiotensin converting enzyme inhibitors and angiotensin receptor blockers: systematic review and meta-analysis. BMJ 345, e4260 (2012)
S.H. Hsia, P. Duran, M.L. Lee, M.B. Davidson, Randomized controlled trial comparing hydroxychloroquine with pioglitazone as third-line agents in type 2 diabetic patients failing metformin plus a Sulfonylurea: A pilot study. J Diabetes 12(1), 91–94 (2020)
F. Malik, S.F. Mehdi, H. Ali et al. Is metformin poised for a second career as an antimicrobial. Diabetes Metab Res Rev 34(4), e2975 (2018)
J. Tuñón, L. Badimón, M.L. Bochaton-Piallat et al. Identifying the anti-inflammatory response to lipid lowering therapy: a position paper from the working group on atherosclerosis and vascular biology of the European Society of Cardiology. Cardiovasc Res 115(1), 10–19 (2019)
H. Roca-Ho, M. Marta Riera, V. Palau et al. Characterization of ACE and ACE2 expression within different organs of the NOD mouse. Int J Mol Sci 18(563), 1–13. (2017)
F.A. Shah, H. Mahmud, T. Gallego-Martin et al. Therapeutic effects of endogenous incretin hormones and exogenous incretin-based medications in sepsis. J Clin Endocrinol Metab 104(11), 5274–5284 (2019)
Acknowledgements
The research and writing of this paper was funded by grants from Special technology for diagnosis and treatment of clinical diseases of Suzhou Health committee (LCZX201707) and Project of Suzhou Medical Key Discipline.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The studies conducted in this article do not involve human participants or animals.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Fang, C., Huang, Y., Guo, H. et al. Mechanism of higher risk for COVID-19 in diabetes: a mask to lift. Endocrine 69, 477–480 (2020). https://doi.org/10.1007/s12020-020-02423-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-020-02423-0