Abstract
Purpose
We aimed to examine the association between leukocyte-related parameters and the risk of metabolic syndrome (MetS) in community-dwelling older Chinese adults, with a special focus on assessing the diagnostic ability of leukocyte-related parameters in detecting MetS and the potential interaction effect of sex in the leukocyte–MetS relationship.
Methods
Study sample was from the Weitang Geriatric Diseases Study, which included 4579 individuals aged 60 years or above. MetS was diagnosed based on the Adult Treatment Panel III criteria. Leukocyte-related parameters were assessed using an automated hematology analyzer.
Results
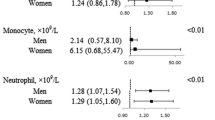
The adjusted odds ratio (95% confidence interval (CI)) of MetS for the highest quartile of leukocyte-related parameters (leukocyte, lymphocyte, neutrophil, monocyte, eosinophil, and basophil), when compared with the lowest quartile were 2.87 (2.30, 3.59), 2.69 (2.15, 3.36), 2.09 (1.67, 2.62), 2.12 (1.71, 2.64), 1.62 (1.31, 2.00), and 1.36 (1.11, 1.65), respectively. Adding leukocyte, lymphocyte, monocyte, and neutrophil to a model containing conventional risk factors improved risk prediction for MetS. Furthermore, significant interactions between leukocyte, monocyte, neutrophil, and sex on MetS were observed (all P value for interaction <0.01).
Conclusion
The numbers of total leukocytes, lymphocyte, monocyte, neutrophil, and eosinophil counts were elevated in older adults with MetS, suggesting that leukocyte-related parameters may be meaningful biomarkers for MetS. Adding leukocyte-related parameters to the conventional models increased the ability of predicting MetS among older adults. These parameters may be useful biomarkers for further risk appraisal of MetS in older adults.


Similar content being viewed by others
References
R.H. Eckel, S.M. Grundy, P.Z. Zimmet, The metabolic syndrome. Lancet 365(9468), 1415–1428 (2005)
J. Lu, L. Wang, M. Li, Y. Xu, Y. Jiang, W. Wang, J. Li, S. Mi, M. Zhang, Y. Li, T. Wang, M. Xu, Z. Zhao, M. Dai, S. Lai, W. Zhao, L. Wang, Y. Bi, G. Ning, Metabolic syndrome among adults in China: the 2010 China Noncommunicable Disease Surveillance. J. Clin. Endocrinol. Metab. 102(2), 507–515 (2017)
B. Isomaa, P. Almgren, T. Tuomi, B. Forsen, K. Lahti, M. Nissen, M.R. Taskinen, L. Groop, Cardiovascular morbidity and mortality associated with the metabolic syndrome. Diabetes Care 24(4), 683–689 (2001)
X. Huo, L. Gao, L. Guo, W. Xu, W. Wang, X. Zhi, L. Li, Y. Ren, X. Qi, Z. Sun, W. Li, Q. Ji, X. Ran, B. Su, C. Hao, J. Lu, X. Guo, H. Zhuo, D. Zhang, C. Pan, J. Weng, D. Hu, X. Yang, L. Ji, Risk of non-fatal cardiovascular diseases in early-onset versus late-onset type 2 diabetes in China: a cross-sectional study. Lancet Diabetes Endocrinol. 4(2), 115–124 (2016)
P. Zimmet, D. Magliano, Y. Matsuzawa, G. Alberti, J. Shaw, The metabolic syndrome: a global public health problem and a new definition. J. Atheroscler. Thromb. 12(6), 295–300 (2005)
P. Dandona, A. Aljada, A. Chaudhuri, P. Mohanty, R. Garg, Metabolic syndrome: a comprehensive perspective based on interactions between obesity, diabetes, and inflammation. Circulation 111(11), 1448–1454 (2005)
G.S. Hotamisligil, Inflammation and metabolic disorders. Nature 444(7121), 860–867 (2006)
W. Meng, C. Zhang, Q. Zhang, X. Song, H. Lin, D. Zhang, Y. Zhang, Z. Zhu, S. Wu, Y. Liu, F. Tang, X. Yang, F. Xue, Association between leukocyte and metabolic syndrome in urban Han Chinese: a longitudinal cohort study. PloS One 7(11), e49875 (2012)
A. Jesri, E.C. Okonofua, B.M. Egan, Platelet and white blood cell counts are elevated in patients with the metabolic syndrome. J. Clin. Hypertens. 7(12), 705–711 (2005)
N. Nagasawa, K. Tamakoshi, H. Yatsuya, Y. Hori, M. Ishikawa, C. Murata, H. Zhang, K. Wada, R. Otsuka, T. Mabuchi, T. Kondo, H. Toyoshima, Association of white blood cell count and clustered components of metabolic syndrome in Japanese men. Circ. J. 68(10), 892–897 (2004)
E. Oda, R. Kawai, The prevalence of metabolic syndrome and diabetes increases through the quartiles of white blood cell count in Japanese men and women. Intern. Med. 48(13), 1127–1134 (2009)
K. Odagiri, A. Uehara, I. Mizuta, M. Yamamoto, C. Kurata, Longitudinal study on white blood cell count and the incidence of metabolic syndrome. Intern. Med. 50(21), 2491–2498 (2011)
D. Fairweather, S. Frisancho-Kiss, N.R. Rose, Sex differences in autoimmune disease from a pathological perspective. Am. J. Pathol. 173(3), 600–609 (2008)
C.W. Pan, X. Wang, Q. Ma, H.P. Sun, Y. Xu, P. Wang, Cognitive dysfunction and health-related quality of life among older Chinese. Sci. Rep. 5, 17301 (2015)
Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III). JAMA 285(19), 2486–2497 (2001). https://doi.org/10.1001/jama.285.19.2486
M.J. Pencina, R.B. D’Agostino, R.B. D’Agostino Sr., R.S. Vasan Jr., Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat. Med. 27(2), 157–172 (2008). https://doi.org/10.1002/sim.2929
B.D. Horne, J.L. Anderson, J.M. John, A. Weaver, T.L. Bair, K.R. Jensen, D.G. Renlund, J.B. Muhlestein, Which white blood cell subtypes predict increased cardiovascular risk? J. Am. Coll. Cardiol. 45(10), 1638–1643 (2005). https://doi.org/10.1016/j.jacc.2005.02.054
P.M. Sweetnam, H.F. Thomas, J.W. Yarnell, I.A. Baker, P.C. Elwood, Total and differential leukocyte counts as predictors of ischemic heart disease: the Caerphilly and Speedwell studies. Am. J. Epidemiol. 145(5), 416–421 (1997). https://doi.org/10.1093/oxfordjournals.aje.a009123
B.Y. Su, C.F. Tian, B.L. Gao, Y.H. Tong, X.H. Zhao, Y. Zheng, Correlation of the leucocyte count with traditional and non-traditional components of metabolic syndrome. Postgrad. Med. 128(8), 805–809 (2016). https://doi.org/10.1080/00325481.2016.1243980
J.A. Kim, Y.S. Choi, J.I. Hong, S.H. Kim, H.H. Jung, S.M. Kim, Association of metabolic syndrome with white blood cell subtype and red blood cells. Endocr. J. 53(1), 133–139 (2006)
H. Yang, Y.Q. Fu, B. Yang, J.S. Zheng, X.Y. Zeng, W. Zeng, Z.F. Fan, M. Chen, L. Wang, D. Li, Positive association between the metabolic syndrome and white blood cell counts in Chinese. Asia Pac. J. Clin. Nutr. 26(1), 141–147 (2017). https://doi.org/10.6133/apjcn.102015.13
X.Q. Lao, G. Neil Thomas, C. Jiang, W. Zhang, P. Adab, T.H. Lam, K.K. Cheng, White blood cell count and the metabolic syndrome in older Chinese: the Guangzhou Biobank Cohort Study. Atherosclerosis 201(2), 418–424 (2008). https://doi.org/10.1016/j.atherosclerosis.2007.12.053
A.E. Kitabchi, M. Temprosa, W.C. Knowler, S.E. Kahn, S.E. Fowler, S.M. Haffner, R. Andres, C. Saudek, S.L. Edelstein, R. Arakaki, M.B. Murphy, H. Shamoon, Role of insulin secretion and sensitivity in the evolution of type 2 diabetes in the diabetes prevention program: effects of lifestyle intervention and metformin. Diabetes 54(8), 2404–2414 (2005). https://doi.org/10.2337/diabetes.54.8.2404
B. Razani, M.V. Chakravarthy, C.F. Semenkovich, Insulin resistance and atherosclerosis. Endocrinol. Metab. Clin. N. Am. 37(3), 603–621, viii (2008). https://doi.org/10.1016/j.ecl.2008.05.001
S. Hagita, M. Osaka, K. Shimokado, M. Yoshida, Adipose inflammation initiates recruitment of leukocytes to mouse femoral artery: role of adipo-vascular axis in chronic inflammation. PloS One 6(5), e19871 (2011). https://doi.org/10.1371/journal.pone.0019871
N. Kawanishi, H. Yano, Y. Yokogawa, K. Suzuki, Exercise training inhibits inflammation in adipose tissue via both suppression of macrophage infiltration and acceleration of phenotypic switching from M1 to M2 macrophages in high-fat-diet-induced obese mice. Exerc. Immunol. Rev. 16, 105–118 (2010)
K. Ohashi, J.L. Parker, N. Ouchi, A. Higuchi, J.A. Vita, N. Gokce, A.A. Pedersen, C. Kalthoff, S. Tullin, A. Sams, R. Summer, K. Walsh, Adiponectin promotes macrophage polarization toward an anti-inflammatory phenotype. J. Biol. Chem. 285(9), 6153–6160 (2010). https://doi.org/10.1074/jbc.M109.088708
K. Li, W. Xu, Q. Guo, Z. Jiang, P. Wang, Y. Yue, S. Xiong, Differential macrophage polarization in male and female BALB/c mice infected with coxsackievirus B3 defines susceptibility to viral myocarditis. Circ. Res. 105(4), 353–364 (2009). https://doi.org/10.1161/circresaha.109.195230
N. Nakanishi, K. Suzuki, K. Tatara, White blood cell count and clustered features of metabolic syndrome in Japanese male office workers. Occup. Med. 52(4), 213–218 (2002). https://doi.org/10.1093/occmed/52.4.213
V. Mohamed-Ali, S. Goodrick, A. Rawesh, D.R. Katz, J.M. Miles, J.S. Yudkin, S. Klein, S.W. Coppack, Subcutaneous adipose tissue releases interleukin-6, but not tumor necrosis factor-alpha, in vivo. J. Clin. Endocrinol. Metab. 82(12), 4196–4200 (1997). https://doi.org/10.1210/jcem.82.12.4450
M. Benagiano, A. Azzurri, A. Ciervo, A. Amedei, C. Tamburini, M. Ferrari, J.L. Telford, C.T. Baldari, S. Romagnani, A. Cassone, M.M. D’Elios, G. Del Prete, T helper type 1 lymphocytes drive inflammation in human atherosclerotic lesions. Proc. Natl Acad. Sci. USA 100(11), 6658–6663 (2003). https://doi.org/10.1073/pnas.1135726100
B. Osterud, Tissue factor/TFPI and blood cells. Thromb. Res. 129(3), 274–278 (2012). https://doi.org/10.1016/j.thromres.2011.11.049
N.P. Kumar, K. Moideen, Y. Bhootra, A. Nancy, V. Viswanathan, B.S. Shruthi, S. Sivakumar, M. Natarajan, H. Kornfeld, S. Babu, Elevated circulating levels of monocyte activation markers among tuberculosis. Immunology 156(3), 249–258 (2019). https://doi.org/10.1111/imm.13023
E. Palella, R. Cimino, S.A. Pullano, A.S. Fiorillo, E. Gulletta, A. Brunetti, D.P. Foti, M. Greco, Laboratory parameters of hemostasis, adhesion molecules, and inflammation in type 2 diabetes mellitus: correlation with glycemic control. Int. J. Environ. Res. Public. Health 17(1), E300 (2020). https://doi.org/10.3390/ijerph17010300
G. Twig, A. Afek, A. Shamiss, E. Derazne, D. Tzur, B. Gordon, A. Tirosh, White blood cells count and incidence of type 2 diabetes in young men. Diabetes Care 36(2), 276–282 (2013). https://doi.org/10.2337/dc11-2298
K.C. Sung, S. Ryu, J.W. Sung, Y.B. Kim, Y.S. Won, D.S. Cho, S.H. Kim, A. Liu, Inflammation in the prediction of type 2 diabetes and hypertension in healthy. Arch. Med. Res. 48(6), 535–545 (2017). https://doi.org/10.1016/j.arcmed.2017.11.010
E.S. Androulakis, D. Tousoulis, N. Papageorgiou, C. Tsioufis, I. Kallikazaros, C. Stefanadis, Essential hypertension: is there a role for inflammatory mechanisms?. Cardiol. Rev. 17(5), 216–221 (2009). https://doi.org/10.1097/CRD.0b013e3181b18e03
D.J. Kim, J.H. Noh, B.W. Lee, Y.H. Choi, J.H. Chung, Y.K. Min, M.S. Lee, M.K. Lee, K.W. Kim, The associations of total and differential white blood cell counts with obesity, hypertension, dyslipidemia and glucose intolerance in a Korean population. J. Korean Med. Sci. 23(2), 193–198 (2008). https://doi.org/10.3346/jkms.2008.23.2.193
V. Lohsoonthorn, W. Jiamjarasrungsi, M.A. Williams, Association of hematological parameters with clustered components of metabolic syndrome among professional and office workers in Bangkok, Thailand. Diabetes Metab. Syndr. 1(3), 143–149 (2007). https://doi.org/10.1016/j.dsx.2007.05.002
P. Zhou, Z. Meng, M. Liu, X. Ren, M. Zhu, Q. He, Q. Zhang, L. Liu, K. Song, Q. Jia, J. Tan, X. Li, N. Liu, T. Hu, A. Upadhyaya, The associations between leukocyte, erythrocyte or platelet, and metabolic syndrome in different genders of Chinese. Medicine 95(44), e5189 (2016). https://doi.org/10.1097/md.0000000000005189
N. Ishizaka, Y. Ishizaka, E. Toda, R. Nagai, M. Yamakado, Association between cigarette smoking, white blood cell count, and metabolic syndrome as defined by the Japanese criteria. Intern. Med. 46(15), 1167–1170 (2007). https://doi.org/10.2169/internalmedicine.46.0136
Y. Ogawa, M. Imaki, Y. Yoshida, M. Shibakawa, S. Tanada, An epidemiological study on the association between the total leukocyte and neutrophil counts, and risk factors of ischemic heart disease by smoking status in Japanese factory workers. Appl. Hum. Sci. 17(6), 239–247 (1998). https://doi.org/10.2114/jpa.17.239
J. Schwartz, S.T. Weiss, Cigarette smoking and peripheral blood leukocyte differentials. Ann. Epidemiol. 4(3), 236–242 (1994). https://doi.org/10.1016/1047-2797(94)90102-3
Y.Y. Cheng, T.W. Kao, Y.W. Chang, C.J. Wu, T.C. Peng, L.W. Wu, H.F. Yang, F.Y. Liaw, W.L. Chen, Examining the gender difference in the association between metabolic syndrome and the mean leukocyte telomere length. PLoS ONE 12(7), e0180687 (2017). https://doi.org/10.1371/journal.pone.0180687
J.H. Kim, J.A. Im, D.C. Lee, The relationship between leukocyte mitochondrial DNA contents and metabolic syndrome in postmenopausal women. Menopause 19(5), 582–587 (2012). https://doi.org/10.1097/gme.0b013e31823a3e46
C. Duffaut, A. Zakaroff-Girard, V. Bourlier, P. Decaunes, M. Maumus, P. Chiotasso, C. Sengenes, M. Lafontan, J. Galitzky, A. Bouloumie, Interplay between human adipocytes and T lymphocytes in obesity: CCL20 as an adipochemokine and T lymphocytes as lipogenic modulators. Arterioscler. Thromb. Vasc. Biol. 29(10), 1608–1614 (2009). https://doi.org/10.1161/atvbaha.109.192583
E. Oda, High-sensitivity C-reactive protein and white blood cell count equally predict development of the metabolic syndrome in a Japanese health screening population. Acta Diabetol. 50(4), 633–638 (2013). https://doi.org/10.1007/s00592-013-0477-7
E. Oda, R. Kawai, Comparison between high-sensitivity C-reactive protein (hs-CRP) and white blood cell count (WBC) as an inflammatory component of metabolic syndrome in Japanese. Intern. Med. 49(2), 117–124 (2010). https://doi.org/10.2169/internalmedicine.49.2670
Acknowledgements
This study was supported by the Science and Technology Bureau of Xiangcheng District in Suzhou, China under grant no. XJ201706 and the Health Commission of Suzhou under grant no. GSWS2019090.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This study was conducted comply with the tenets of the Helsinki Declaration, and with the approval of the Institutional Review Board of Soochow University.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yang, XJ., Tian, S., Ma, QH. et al. Leukocyte-related parameters in older adults with metabolic syndrome. Endocrine 68, 312–319 (2020). https://doi.org/10.1007/s12020-020-02243-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-020-02243-2