Abstract
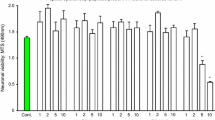
We have demonstrated that arginine-rich and poly-arginine peptides possess potent neuroprotective properties with arginine content and peptide positive charge being particularly critical for neuroprotective efficacy. In addition, the presence of other amino acids within arginine-rich peptides, as well as chemical modifications, peptide length and cell-penetrating properties also influence the level of neuroprotection. Against this background, we have examined the neuroprotective efficacy of arginine-rich protamine peptides, a cyclic (R12-c) poly-arginine peptide and a R22 poly-arginine peptide, as well as arginine peptides containing tryptophan or other amino acids (phenylalanine, tyrosine, glycine or leucine) in in vitro glutamic acid excitotoxicity and in vivo rat permanent middle cerebral artery occlusion models of stroke. In vitro studies demonstrated that protamine and poly-arginine peptides (R12-c, R22) were neuroprotective. Arginine–tryptophan-containing peptides were highly neuroprotective, with R12W8a being the most potent arginine-rich peptide identified in our laboratory. Peptides containing phenylalanine or tyrosine substituted in place of tryptophan in R12W8a were also highly neuroprotective, whereas leucine, and in particular glycine substitutions, decreased peptide efficacy. In vivo studies with protamine administered intravenously at 1000 nmol/kg 30 min after MCAO significantly reduced infarct volume and cerebral oedema by 22.5 and 38.6%, respectively. The R12W8a peptide was highly toxic when administered intravenously at 300 or 100 nmol/kg and ineffective at reducing infarct volume when administered at 30 nmol/kg 30 min after MCAO, unlike R18 (30 nmol/kg), which significantly reduced infarct volume by 20.4%. However, both R12W8a and R18 significantly reduced cerebral oedema by 19.8 and 42.2%, respectively. Protamine, R12W8a and R18 also reduced neuronal glutamic acid-induced calcium influx. These findings further highlight the neuroprotective properties of arginine-rich peptides and support the view that they represent a new class of neuroprotective agent.







Similar content being viewed by others
References
Bechara, C., Pallerla, M., Burlina, F., Illien, F., Cribier, S., & Sagan, S. (2015). Massive glycosaminoglycan-dependent entry of Trp-containing cell-penetrating peptides induced by exogenous sphingomyelinase or cholesterol depletion. Cellular and Molecular Life Sciences, 72(4), 809–820.
Bechara, C., Pallerla, M., Zaltsman, Y., Burlina, F., Alves, I. D., Lequin, O., et al. (2013). Tryptophan within basic peptide sequences triggers glycosaminoglycan-dependent endocytosis. FASEB Journal, 27(2), 738–749.
Brittain, J. M., Chen, L., Wilson, S. M., Brustovetsky, T., Gao, X., Ashpole, N. M., et al. (2011). Neuroprotection against traumatic brain injury by a peptide derived from the collapsin response mediator protein 2 (CRMP2). Journal of Biological Chemistry, 286(43), 37778–37792.
Brustovetsky, T., Pellman, J. J., Yang, X. F., Khanna, R., & Brustovetsky, N. (2014). Collapsin response mediator protein 2 (CRMP2) interacts with N-methyl-D-aspartate (NMDA) receptor and Na+/Ca2+ exchanger and regulates their functional activity. Journal of Biological Chemistry, 289(11), 7470–7482.
Byun, Y., Singh, V. K., & Yang, V. C. (1999). Low molecular weight protamine: A potential nontoxic heparin antagonist. Thrombosis Research, 94(1), 53–61.
Campbell, K., Meloni, B. P., & Knuckey, N. W. (2008). Combined magnesium and mild hypothermia (35°C) treatment reduces infarct volumes after permanent middle cerebral artery occlusion in the rat at 2 and 4, but not 6 hours. Brain Research, 1230, 258–264.
DeLucia, A., 3rd, Wakefield, T. W., Andrews, P. C., Nichol, B. J., Kadell, A. M., Wrobleski, S. K., et al. (1993). Efficacy and toxicity of differently charged polycationic protamine-like peptides for heparin anticoagulation reversal. Journal of Vascular Surgery, 18(1), 49–58.
Diaz-Sylvester, P. L., & Copello, J. A. (2009). Voltage-dependent modulation of cardiac ryanodine receptors (RyR2) by protamine. PLoS ONE, 4(12), e8315. doi:10.1371/journal.pone.0008315.
Ferrer-Montiel, A. V., Merino, J. M., Blondelle, S. E., Perez-Paya, E., Houghten, R. A., & Montal, M. (1998). Selected peptides targeted to the NMDA receptor channel protect neurons from excitotoxic death. Nature Biotechnology, 16(3), 286–291.
Fotin-Mleczek, M., Welte, S., Mader, O., Duchardt, F., Fischer, R., Hufnagel, H., et al. (2005). Cationic cell-penetrating peptides interfere with TNF signalling by induction of TNF receptor internalization. Journal of Cell Science, 118(Pt 15), 3339–3351.
He, H., Ye, J., Liu, E., Liang, Q., Liu, Q., & Yang, V. C. (2014). Low molecular weight protamine (LMWP): A nontoxic protamine substitute and an effective cell-penetrating peptide. Journal of Controlled Release, 193, 63–73.
Henninger, N., & Fisher, M. (2016). Extending the time window for endovascular and pharmacological reperfusion. Translational Stroke Research, 7(4), 284–293.
Hoffmann, J. A., Chance, R. E., & Johnson, M. G. (1990). Purification and analysis of the major components of chum salmon protamine contained in insulin formulations using high-performance liquid chromatography. Protein Expression and Purification, 1(2), 127–133.
Hoque, A., Hossain, M. I., Ameen, S. S., Ang, C. S., Williamson, N., Ng, D. C., et al. (2016). A beacon of hope in stroke therapy—Blockade of pathologically activated cellular events in excitotoxic neuronal death as potential neuroprotective strategies. Pharmacology and Therapeutics, 160, 159–179.
Horrow, J. C. (1985). Protamine: A review of its toxicity. Anesthesia and Analgesia, 64(3), 348–361.
Jobin, M. L., Blanchet, M., Henry, S., Chaignepain, S., Manigand, C., Castano, S., et al. (2015). The role of tryptophans on the cellular uptake and membrane interaction of arginine-rich cell penetrating peptides. Biochimica et Biophysica Acta, 1848(2), 593–602.
Koulen, P., & Ehrlich, B. E. (2000). Reversible block of the calcium release channel/ryanodine receptor by protamine, a heparin antidote. Molecular Biology of the Cell, 11(7), 2213–2219.
Li, T., Meng, Z., Zhu, X., Gan, H., Gu, R., Wu, Z., et al. (2015). New synthetic peptide with efficacy for heparin reversal and low toxicity and immunogenicity in comparison to protamine sulfate. Biochemical and Biophysical Research Communications, 467(3), 497–502.
MacDougall, G., Anderton, R. S., Edwards, A. B., Knuckey, N. W., & Meloni, B. P. (2017). The neuroprotective peptide poly-arginine-12 (R12) reduces cell surface levels of NMDA NR2B receptor subunit in cortical neurons; investigation into the involvement of endocytic mechanisms. Journal of Molecular Neuroscience, 61, 235–246.
Marshall, J., Wong, K. Y., Rupasinghe, C. N., Tiwari, R., Zhao, X., Berberoglu, E. D., et al. (2015). Inhibition of N-methyl-D-aspartate-induced retinal neuronal death by polyarginine peptides is linked to the attenuation of stress-induced hyperpolarization of the inner mitochondrial membrane potential. Journal of Biological Chemistry, 290(36), 22030–22048.
Matsunaga, M., Ohtaki, H., Takaki, A., Iwai, Y., Yin, L., Mizuguchi, H., et al. (2003). Nucleoprotamine diet derived from salmon soft roe protects mouse hippocampal neurons from delayed cell death after transient forebrain ischemia. Neuroscience Research, 47(3), 269–276.
Mehta, C. R., & Patel, N. R. (2006). Adaptive, group sequential and decision theoretic approaches to sample size determination. Statistics in Medicine, 25(19), 3250–3269.
Meloni, B. P., Brookes, L. M., Clark, V. W., Cross, J. L., Edwards, A. B., Anderton, R. S., et al. (2015a). Poly-arginine and arginine-rich peptides are neuroprotective in stroke models. Journal of Cerebral Blood Flow and Metabolism, 35(6), 993–1004.
Meloni, B. P., Craig, A. J., Milech, N., Hopkins, R. M., Watt, P. M., & Knuckey, N. W. (2014). The neuroprotective efficacy of cell-penetrating peptides TAT, penetratin, Arg-9, and Pep-1 in glutamic acid, kainic acid, and in vitro ischemia injury models using primary cortical neuronal cultures. Cellular and Molecular Neurobiology, 34(2), 173–181.
Meloni, B. P., Milani, D., Edwards, A. B., Anderton, R. S., O’Hare, Doig R. L., Fitzgerald, M., et al. (2015b). Neuroprotective peptides fused to arginine-rich cell penetrating peptides: Neuroprotective mechanism likely mediated by peptide endocytic properties. Pharmacology and Therapeutics, 153, 36–54.
Milani, D., Clark, V. W., Cross, J. L., Anderton, R. S., Knuckey, N. W., & Meloni, B. P. (2016a). Poly-arginine peptides reduce infarct volume in a permanent middle cerebral artery rat stroke model. BMC Neuroscience, 17(1), 1–8.
Milani, D., Cross, J. L., Knuckey, N. W., Blacker, D. J., Anderton, R. S., & Meloni, B. P. (2017). Neuroprotective efficacy of R18 poly-arginine and NA-1 (TAT-NR2B9c) peptides following transient middle cerebral artery occlusion in the rat. Neuroscience Research, 114, 9–15.
Milani, D., Knuckey, N. W., Anderton, R. S., Cross, J. L., & Meloni, B. P. (2016b). The R18 poly-arginine peptide is more effective than the TAT–NR2B9c (NA-1) peptide when administered 60 minutes after permanent middle cerebral artery occlusion in the rat. Stroke Research and Treatment, 2016, 1–9.
Mitchell, D. J., Kim, D. T., Steinman, L., Fathman, C. G., & Rothbard, J. B. (2000). Polyarginine enters cells more efficiently than other polycationic homopolymers. Journal of Peptide Research, 56(5), 318–325.
Moutal, A., Francois-Moutal, L., Brittain, J. M., Khanna, M., & Khanna, R. (2014). Differential neuroprotective potential of CRMP2 peptide aptamers conjugated to cationic, hydrophobic, and amphipathic cell penetrating peptides. Frontiers in Cellular Neuroscience. doi:10.3389/fncel.2014.00471.
Oh, D., Nasrolahi Shirazi, A., Northup, K., Sullivan, B., Tiwari, R. K., Bisoffi, M., et al. (2014). Enhanced cellular uptake of short polyarginine peptides through fatty acylation and cyclization. Molecular Pharmacology, 11(8), 2845–2854.
Pevni, D., Gurevich, J., Frolkis, I., Keren, G., Shapira, I., Paz, J., et al. (2000). Protamine induces vasorelaxation of human internal thoracic artery by endothelial NO-synthase pathway. Annals of Thoracic Surgery, 70(6), 2050–2053.
Qian, Z., Martyna, A., Hard, R. L., Wang, J., Appiah-Kubi, G., Coss, C., et al. (2016). discovery and mechanism of highly efficient cyclic cell-penetrating peptides. Biochemistry, 55(18), 2601–2612.
Raikar, G. V., Hisamochi, K., Raikar, B. L., & Schaff, H. V. (1996). Nitric oxide inhibition attenuates systemic hypotension produced by protamine. Journal of Thoracic and Cardiovascular Surgery, 111(6), 1240–1247.
Ruiz, A., Matute, C., & Alberdi, E. (2009). Endoplasmic reticulum Ca2+ release through ryanodine and IP(3) receptors contributes to neuronal excitotoxicity. Cell Calcium, 46(4), 273–281.
Rydberg, H. A., Matson, M., Amand, H. L., Esbjörner, E. K., & Nordén, B. (2012). Effects of tryptophan content and backbone spacing on the uptake efficiency of cell-penetrating peptides. Biochemistry, 51(27), 5531–5539.
Starbuck, W. C., Seibert, R. A., Schwartz, A., Mauritzen, C., Taylor, C. W., & Busch, H. (1967). Studies on the pharmacology and toxicology of histones. Archives Internationales de Pharmacodynamie et de Thérapie, 165(2), 374–383.
Traboulsi, H., Larkin, H., Bonin, M. A., Volkov, L., Lavoie, C. L., & Marsault, É. (2015). Macrocyclic cell penetrating peptides: A study of structure-penetration properties. Bioconjugate Chemistry, 26(3), 405–411.
Tu, W., Xu, X., Peng, L., Zhong, X., Zhang, W., Soundarapandian, M. M., et al. (2010). DAPK1 interaction with NMDA receptor NR2B subunits mediates brain damage in stroke. Cell, 140(2), 222–234.
Tymianski, M. (2014). Stroke in 2013: Disappointments and advances in acute stroke intervention. Nature Reviews Neurology, 10(2), 66–68.
Walrant, A., Vogel, A., Correia, I., Lequin, O., Olausson, B. E., Desbat, B., et al. (2012). Membrane interactions of two arginine-rich peptides with different cell internalization capacities. Biochimica et Biophysica Acta, 1818(7), 1755–1763.
Acknowledgements
This study has been supported by the University of Notre Dame Australia, the Perron Institute, the Department of Neurosurgery at Sir Charles Gairdner Hospital, a Neurotrauma Research Program of Western Australia research grant and Stroke Foundation (Australia) grant. We thank Prof Norman Palmer editorial changes and suggestions to manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Bruno P. Meloni and Neville W. Knuckey are the holders of several patents regarding the use of arginine-rich peptides as neuroprotective treatments. The other authors declare no conflict of interest.
Animal Rights
All animal studies were approved by the University of Western Australia Animal Ethics Committee, and experiments were carried out according to guidelines outlined by the Australian Code for the Care and Use of Animals for Scientific Purposes.
Rights and permissions
About this article
Cite this article
Meloni, B.P., Milani, D., Cross, J.L. et al. Assessment of the Neuroprotective Effects of Arginine-Rich Protamine Peptides, Poly-Arginine Peptides (R12-Cyclic, R22) and Arginine–Tryptophan-Containing Peptides Following In Vitro Excitotoxicity and/or Permanent Middle Cerebral Artery Occlusion in Rats. Neuromol Med 19, 271–285 (2017). https://doi.org/10.1007/s12017-017-8441-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12017-017-8441-2