Abstract
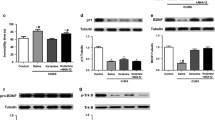
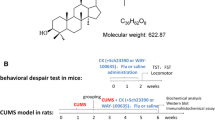
Activation of translocator protein (18 kDa) (TSPO) plays an important role to mediate rapid anxiolytic efficacy in stress response and stress-related disorders by the production of neurosteroids. However, little is known about the ligand of TSPO on the anxiety-like and depressive behaviors and the underlying mechanisms in chronic unpredictable mild stress (UCMS) mice. In the present study, a novel ligand of TSPO, ZBD-2 [N-benzyl-N-ethyl-2-(7,8-dihydro-7-benzyl-8-oxo-2-phenyl-9H-purin-9-yl) acetamide] synthesized by our laboratory, was used to evaluate the anxiolytic and antidepressant efficacy and to elucidate the underlying mechanisms. ZBD-2 (3 mg/kg) significantly attenuated anxiety-like and depressive behaviors in the UCMS mice, which was blocked by TSPO antagonist PK11195 (3 mg/kg). Treatment of ZBD-2 reversed the decrease in biogenic amines (norepinephrine, dopamine, and serotonin) in the brain region of hippocampus in the UCMS mice. The decreases in TSPO, GluN2B-containing N-methyl-d-aspartate (NMDA) receptors, GluA1, p-GluA1-Ser831, p-GluA1-Ser845, PSD-95, and GABAA-a2 were integrated with the increases of CaMKII and iNOS levels in the hippocampus of the UCMS mice. ZBD-2 significantly reversed the changes of above proteins. However, ZBD-2 or PK11195 treatment did not affect the levels of GluN2A-containing NMDA receptors and the total levels of GAD67. Our study provides strong evidences that ZBD-2 has a therapeutic effect on chronic stress-related disorders such as depression and anxiety through regulating the biogenic amine levels and the synaptic proteins in the hippocampus.






Similar content being viewed by others
Abbreviations
- 5-HT:
-
Serotonin
- AMPAR:
-
α-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor
- CNS:
-
Central nervous system
- DA:
-
Dopamine
- EPM:
-
Elevated plus maze
- FST:
-
Forced swim test
- GABA:
-
γ-Aminobutyric acid
- NE:
-
Norepinephrine
- NMDA:
-
N-Methyl-d-aspartate
- OF:
-
Open field
- SSRIs:
-
Selective serotonin reuptake inhibitors
- TSPO:
-
Translocator protein (18 kDa)
- TST:
-
Tail suspension test
- UCMS:
-
Chronic unpredictable mild stress
- ZBD-2:
-
N-Benzyl-N-ethyl-2-(7,8-dihydro-7-benzyl-8-oxo-2-phenyl-9H-purin-9-yl) acetamide
References
Albert, P. R., Vahid-Ansari, F., & Luckhart, C. (2014). Serotonin-prefrontal cortical circuitry in anxiety and depression phenotypes: Pivotal role of pre- and post-synaptic 5-HT1A receptor expression. Frontiers in Behavioral Neuroscience, 8, 199. doi:10.3389/fnbeh.2014.00199.
Anderson, I. M., Nutt, D. J., & Deakin, J. F. (2000). Evidence-based guidelines for treating depressive disorders with antidepressants: A revision of the 1993 British Association for Psychopharmacology guidelines. British Association for Psychopharmacology. Journal of Psychopharmacology, 14(1), 3–20.
Anholt, R. R., Pedersen, P. L., De Souza, E. B., & Snyder, S. H. (1986). The peripheral-type benzodiazepine receptor. Localization to the mitochondrial outer membrane. Journal of Biological Chemistry, 261(2), 576–583.
Belelli, D., & Lambert, J. J. (2005). Neurosteroids: Endogenous regulators of the GABA(A) receptor. Nature Reviews Neuroscience, 6(7), 565–575. doi:10.1038/nrn1703.
Bourin, M., Mocaer, E., & Porsolt, R. (2004). Antidepressant-like activity of S 20098 (agomelatine) in the forced swimming test in rodents: Involvement of melatonin and serotonin receptors. Journal of Psychiatry and Neuroscience, 29(2), 126–133.
Cates, L. N., Roberts, A. J., Huitron-Resendiz, S., & Hedlund, P. B. (2013). Effects of lurasidone in behavioral models of depression. Role of the 5-HT(7) receptor subtype. Neuropharmacology, 70, 211–217. doi:10.1016/j.neuropharm.2013.01.023.
Celada, P., Puig, M., Amargos-Bosch, M., Adell, A., & Artigas, F. (2004). The therapeutic role of 5-HT1A and 5-HT2A receptors in depression. Journal of Psychiatry and Neuroscience, 29(4), 252–265.
De Benedetto, G. E., Fico, D., Pennetta, A., Malitesta, C., Nicolardi, G., Lofrumento, D. D., et al. (2014). A rapid and simple method for the determination of 3,4-dihydroxyphenylacetic acid, norepinephrine, dopamine, and serotonin in mouse brain homogenate by HPLC with fluorimetric detection. Journal of Pharmaceutical and Biomedical Analysis, 98, 266–270. doi:10.1016/j.jpba.2014.05.039.
Dranovsky, A., & Hen, R. (2006). Hippocampal neurogenesis: Regulation by stress and antidepressants. Biological Psychiatry, 59(12), 1136–1143. doi:10.1016/j.biopsych.2006.03.082.
Du, J., Szabo, S. T., Gray, N. A., & Manji, H. K. (2004). Focus on CaMKII: A molecular switch in the pathophysiology and treatment of mood and anxiety disorders. International Journal of Neuropsychopharmacology, 7(3), 243–248. doi:10.1017/S1461145704004432.
Elhwuegi, A. S. (2004). Central monoamines and their role in major depression. Progress in Neuropsychopharmacology and Biological Psychiatry, 28(3), 435–451. doi:10.1016/j.pnpbp.2003.11.018.
Fasick, V., Spengler, R. N., Samankan, S., Nader, N. D., & Ignatowski, T. A. (2015). The hippocampus and TNF: Common links between chronic pain and depression. Neuroscience and Biobehavioral Reviews, 53, 139–159. doi:10.1016/j.neubiorev.2015.03.014.
Gavish, M., Bachman, I., Shoukrun, R., Katz, Y., Veenman, L., Weisinger, G., et al. (1999). Enigma of the peripheral benzodiazepine receptor. Pharmacological Reviews, 51(4), 629–650.
Gavish, M., Laor, N., Bidder, M., Fisher, D., Fonia, O., Muller, U., et al. (1996). Altered platelet peripheral-type benzodiazepine receptor in posttraumatic stress disorder. Neuropsychopharmacology, 14(3), 181–186. doi:10.1016/0893-133X(95)00078-R.
Girard, C., Liu, S., Adams, D., Lacroix, C., Sineus, M., Boucher, C., et al. (2012). Axonal regeneration and neuroinflammation: Roles for the translocator protein 18 kDa. Journal of Neuroendocrinology, 24(1), 71–81. doi:10.1111/j.1365-2826.2011.02215.x.
Guo, Y. Y., Liu, S. B., Cui, G. B., Ma, L., Feng, B., Xing, J. H., et al. (2012). Acute stress induces down-regulation of large-conductance Ca2+-activated potassium channels in the lateral amygdala. Journal of Physiology, 590(Pt 4), 875–886. doi:10.1113/jphysiol.2011.223784.
Haase, J., & Brown, E. (2015). Integrating the monoamine, neurotrophin and cytokine hypotheses of depression—A central role for the serotonin transporter? Pharmacology & Therapeutics, 147, 1–11. doi:10.1016/j.pharmthera.2014.10.002.
Ibarguen-Vargas, Y., Surget, A., Vourc’h, P., Leman, S., Andres, C. R., Gardier, A. M., et al. (2009). Deficit in BDNF does not increase vulnerability to stress but dampens antidepressant-like effects in the unpredictable chronic mild stress. Behavioural Brain Research, 202(2), 245–251. doi:10.1016/j.bbr.2009.03.040.
Kallarackal, A. J., Kvarta, M. D., Cammarata, E., Jaberi, L., Cai, X., Bailey, A. M., et al. (2013). Chronic stress induces a selective decrease in AMPA receptor-mediated synaptic excitation at hippocampal temporoammonic-CA1 synapses. Journal of Neuroscience, 33(40), 15669–15674. doi:10.1523/JNEUROSCI.2588-13.2013.
Khoshnoodi, M., Fakhraei, N., & Dehpour, A. R. (2015). Possible involvement of nitric oxide in antidepressant-like effect of silymarin in male mice. Pharmaceutical Biology, 53(5), 739–745. doi:10.3109/13880209.2014.942787.
Kita, A., Kohayakawa, H., Kinoshita, T., Ochi, Y., Nakamichi, K., Kurumiya, S., et al. (2004). Antianxiety and antidepressant-like effects of AC-5216, a novel mitochondrial benzodiazepine receptor ligand. British Journal of Pharmacology, 142(7), 1059–1072. doi:10.1038/sj.bjp.0705681.
Koo, J. W., & Duman, R. S. (2008). IL-1beta is an essential mediator of the antineurogenic and anhedonic effects of stress. Proceedings of the National Academy of Sciences, 105(2), 751–756. doi:10.1073/pnas.0708092105.
Li, X. B., Guo, H. L., Shi, T. Y., Yang, L., Wang, M., Zhang, K., et al. (2015). Neuroprotective effects of a novel translocator protein (18 kDa) ligand, ZBD-2, against focal cerebral ischemia in vivo and NMDA-induced neurotoxicity in vitro. Clinical and Experimental Pharmacology and Physiology. doi:10.1111/1440-1681.12460.
Li, K., Zhou, T., Liao, L., Yang, Z., Wong, C., Henn, F., et al. (2013). betaCaMKII in lateral habenula mediates core symptoms of depression. Science, 341(6149), 1016–1020. doi:10.1126/science.1240729.
Liang, B. F., Huang, F., Wang, H. T., Wang, G. H., Yuan, X., Zhang, M. Z., et al. (2015). Involvement of norepinephrine and serotonin system in antidepressant-like effects of hederagenin in the rat model of unpredictable chronic mild stress-induced depression. Pharmaceutical Biology, 53(3), 368–377. doi:10.3109/13880209.2014.922586.
Liu, B., Xu, C., Wu, X., Liu, F., Du, Y., Sun, J., et al. (2015). Icariin exerts an antidepressant effect in an unpredictable chronic mild stress model of depression in rats and is associated with the regulation of hippocampal neuroinflammation. Neuroscience, 294, 193–205. doi:10.1016/j.neuroscience.2015.02.053.
Lothe, A., Saoud, M., Bouvard, S., Redoute, J., Lerond, J., & Ryvlin, P. (2012). 5-HT(1A) receptor binding changes in patients with major depressive disorder before and after antidepressant treatment: A pilot [(1)(8)F]MPPF positron emission tomography study. Psychiatry Research, 203(1), 103–104. doi:10.1016/j.pscychresns.2011.09.001.
Murata, Y., Yanagihara, Y., Mori, M., Mine, K., & Enjoji, M. (2015). Chronic treatment with tandospirone, a serotonin 1A receptor partial agonist, inhibits psychosocial stress-induced changes in hippocampal neurogenesis and behavior. Journal of Affective Disorders, 180, 1–9. doi:10.1016/j.jad.2015.03.054.
Myers, K. M., Carlezon, W. A, Jr., & Davis, M. (2011). Glutamate receptors in extinction and extinction-based therapies for psychiatric illness. Neuropsychopharmacology, 36(1), 274–293. doi:10.1038/npp.2010.88.
Nelson, E. M., & Philbrick, A. M. (2012). Avoiding serotonin syndrome: The nature of the interaction between tramadol and selective serotonin reuptake inhibitors. Annals of Pharmacotherapy, 46(12), 1712–1716. doi:10.1345/aph.1Q748.
Nothdurfter, C., Baghai, T. C., Schule, C., & Rupprecht, R. (2012). Translocator protein (18 kDa) (TSPO) as a therapeutic target for anxiety and neurologic disorders. European Archives of Psychiatry and Clinical Neuroscience, 262(Suppl 2), S107–S112. doi:10.1007/s00406-012-0352-5.
Papadopoulos, V., Lecanu, L., Brown, R. C., Han, Z., & Yao, Z. X. (2006). Peripheral-type benzodiazepine receptor in neurosteroid biosynthesis, neuropathology and neurological disorders. Neuroscience, 138(3), 749–756. doi:10.1016/j.neuroscience.2005.05.063.
Rocca, P., Beoni, A. M., Eva, C., Ferrero, P., Zanalda, E., & Ravizza, L. (1998). Peripheral benzodiazepine receptor messenger RNA is decreased in lymphocytes of generalized anxiety disorder patients. Biological Psychiatry, 43(10), 767–773.
Rupprecht, R., Rammes, G., Eser, D., Baghai, T. C., Schule, C., Nothdurfter, C., et al. (2009). Translocator protein (18 kD) as target for anxiolytics without benzodiazepine-like side effects. Science, 325(5939), 490–493. doi:10.1126/science.1175055.
Schmidt, M. V., Trumbach, D., Weber, P., Wagner, K., Scharf, S. H., Liebl, C., et al. (2010). Individual stress vulnerability is predicted by short-term memory and AMPA receptor subunit ratio in the hippocampus. Journal of Neuroscience, 30(50), 16949–16958. doi:10.1523/JNEUROSCI.4668-10.2010.
Suenaga, T., Morinobu, S., Kawano, K., Sawada, T., & Yamawaki, S. (2004). Influence of immobilization stress on the levels of CaMKII and phospho-CaMKII in the rat hippocampus. International Journal of Neuropsychopharmacology, 7(3), 299–309. doi:10.1017/S1461145704004304.
Sullivan, G. M., Oquendo, M. A., Milak, M., Miller, J. M., Burke, A., Ogden, R. T., et al. (2015). Positron emission tomography quantification of serotonin(1A) receptor binding in suicide attempters with major depressive disorder. JAMA Psychiatry, 72(2), 169–178. doi:10.1001/jamapsychiatry.2014.2406.
Tian, Z., Wang, Y., Zhang, N., Guo, Y. Y., Feng, B., Liu, S. B., et al. (2013). Estrogen receptor GPR30 exerts anxiolytic effects by maintaining the balance between GABAergic and glutamatergic transmission in the basolateral amygdala of ovariectomized mice after stress. Psychoneuroendocrinology, 38(10), 2218–2233. doi:10.1016/j.psyneuen.2013.04.011.
Tomaz, V. S., Cordeiro, R. C., Costa, A. M., de Lucena, D. F., Nobre Junior, H. V., de Sousa, F. C., et al. (2014). Antidepressant-like effect of nitric oxide synthase inhibitors and sildenafil against lipopolysaccharide-induced depressive-like behavior in mice. Neuroscience, 268, 236–246. doi:10.1016/j.neuroscience.2014.03.025.
Vasquez, C. E., Riener, R., Reynolds, E., & Britton, G. B. (2014). NMDA receptor dysregulation in chronic state: A possible mechanism underlying depression with BDNF downregulation. Neurochemistry International, 79, 88–97. doi:10.1016/j.neuint.2014.09.007.
Veenman, L., & Gavish, M. (2006). The peripheral-type benzodiazepine receptor and the cardiovascular system. Implications for drug development. Pharmacology & Therapeutics, 110(3), 503–524. doi:10.1016/j.pharmthera.2005.09.007.
Wang, D. S., Tian, Z., Guo, Y. Y., Guo, H. L., Kang, W. B., Li, S., et al. (2015). Anxiolytic-like effects of translocator protein (TSPO) ligand ZBD-2 in an animal model of chronic pain. Molecular Pain, 11, 16. doi:10.1186/s12990-015-0013-6.
Weizman, R., & Gavish, M. (1993). Molecular cellular and behavioral aspects of peripheral-type benzodiazepine receptors. Clinical Neuropharmacology, 16(5), 401–417.
Willner, P., Towell, A., Sampson, D., Sophokleous, S., & Muscat, R. (1987). Reduction of sucrose preference by chronic unpredictable mild stress, and its restoration by a tricyclic antidepressant. Psychopharmacology (Berl), 93(3), 358–364.
Zhu, S., Wang, J., Zhang, Y., Li, V., Kong, J., He, J., et al. (2014). Unpredictable chronic mild stress induces anxiety and depression-like behaviors and inactivates AMP-activated protein kinase in mice. Brain Research, 1576, 81–90. doi:10.1016/j.brainres.2014.06.002.
Zorumski, C. F., Paul, S. M., Izumi, Y., Covey, D. F., & Mennerick, S. (2013). Neurosteroids, stress and depression: Potential therapeutic opportunities. Neuroscience and Biobehavioral Reviews, 37(1), 109–122. doi:10.1016/j.neubiorev.2012.10.005.
Acknowledgments
This work was supported by the National Natural Science Foundation of China Nos. 81325022, 31470052, 2011ZXJ09106-01C, and Clinical Science and Technology Project Funding in Jiangsu Province (No. BL2012002).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Dong-sheng Wang, Jing Han and Shuo Li have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Wang, Ds., Han, J., Li, S. et al. Antidepressant-Like and Anxiolytic-Like Effects of ZBD-2, a Novel Ligand for the Translocator Protein (18 kDa). Neuromol Med 19, 57–68 (2017). https://doi.org/10.1007/s12017-016-8425-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12017-016-8425-7