Abstract
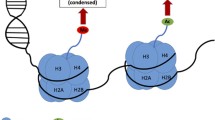
Synaptic plasticity is one of the most fundamental properties of neurons that underlie the formation of the memory in brain. In recent years, epigenetic modification of both DNA and histones such as DNA methylation and histone acetylation and methylation emerges as a potential regulatory mechanism that governs the transcription of several genes responsible for memory formation and behavior. Furthermore, the recent identification of nitrosylation of proteins has shown to either activate or repress gene transcription by modulating histone methylation or acetylation status in mature neuron. Recent studies suggest that the use of major substrates of abuse, e.g., cocaine, induces alterations in molecular and cellular mechanisms of epigenetics that underlie long-term memories in the striatum and prefrontal cortex. Moreover, downregulation of genes due to alterations in epigenetics leads to cognitive deficiencies associated with neurological disorders such as Alzheimer’s disease, Huntington’s disease, psychiatric disorder such as Rett’s syndrome and aging. In this review, I will discuss the evidence for several epigenetic mechanisms in the coordination of complex memory formation and storage. In addition, I will address the current literature highlighting the role of acetylation and methylation of chromatin in memory impairment associated with several neurological disorders, aging, and addiction.


Similar content being viewed by others
References
Abel, T., & Lattal, K. M. (2001). Molecular mechanisms of memory acquisition, consolidation and retrieval. Current Opinion in Neurobiology, 11(2), 180–187.
Afsari, K., Frank, J., Vaksman, Y., & Nguyen, T. V. (2003). Intracranial venous sinus thrombosis complicating AIDS-associated nephropathy. The AIDS Reader, 13(3), 143–148.
Aguilar-Valles, A., Vaissiere, T., Griggs, E. M., Mikaelsson, M. A., Takacs, I. F., Young, E. J., et al. (2013). Methamphetamine-associated memory is regulated by a writer and an eraser of permissive histone methylation. Biological Psychiatry,. doi:10.1016/j.biopsych.2013.09.014.
Alarcon, J. M., Malleret, G., Touzani, K., Vronskaya, S., Ishii, S., Kandel, E. R., et al. (2004). Chromatin acetylation, memory, and LTP are impaired in CBP±mice: A model for the cognitive deficit in Rubinstein–Taybi syndrome and its amelioration. Neuron, 42(6), 947–959. doi:10.1016/j.neuron.2004.05.021.
Alberini, C. M. (2009). Transcription factors in long-term memory and synaptic plasticity. Physiological Reviews, 89(1), 121–145. doi:10.1152/physrev.00017.2008.
Archibald, K., Perry, M. J., Molnar, E., & Henley, J. M. (1998). Surface expression and metabolic half-life of AMPA receptors in cultured rat cerebellar granule cells. Neuropharmacology, 37(10–11), 1345–1353.
Bahari-Javan, S., Maddalena, A., Kerimoglu, C., Wittnam, J., Held, T., Bahr, M., et al. (2012). HDAC1 regulates fear extinction in mice. Journal of Neuroscience, 32(15), 5062–5073. doi:10.1523/JNEUROSCI.0079-12.2012.
Berger, S. L. (2007). The complex language of chromatin regulation during transcription. Nature, 447(7143), 407–412. doi:10.1038/nature05915.
Bertran-Gonzalez, J., Bosch, C., Maroteaux, M., Matamales, M., Herve, D., Valjent, E., et al. (2008). Opposing patterns of signaling activation in dopamine D1 and D2 receptor-expressing striatal neurons in response to cocaine and haloperidol. Journal of Neuroscience, 28(22), 5671–5685. doi:10.1523/JNEUROSCI.1039-08.2008.
Borrelli, E., Nestler, E. J., Allis, C. D., & Sassone-Corsi, P. (2008). Decoding the epigenetic language of neuronal plasticity. Neuron, 60(6), 961–974. doi:10.1016/j.neuron.2008.10.012.
Bredt, D. S., & Snyder, S. H. (1994). Transient nitric oxide synthase neurons in embryonic cerebral cortical plate, sensory ganglia, and olfactory epithelium. Neuron, 13(2), 301–313.
Bredy, T. W., Wu, H., Crego, C., Zellhoefer, J., Sun, Y. E., & Barad, M. (2007). Histone modifications around individual BDNF gene promoters in prefrontal cortex are associated with extinction of conditioned fear. Learning & Memory, 14(4), 268–276. doi:10.1101/lm.500907.
Broide, R. S., Redwine, J. M., Aftahi, N., Young, W., Bloom, F. E., & Winrow, C. J. (2007). Distribution of histone deacetylases 1-11 in the rat brain. Journal of Molecular Neuroscience, 31(1), 47–58.
Carlezon, W. A, Jr, Duman, R. S., & Nestler, E. J. (2005). The many faces of CREB. Trends in Neurosciences, 28(8), 436–445. doi:10.1016/j.tins.2005.06.005.
Cassel, S., Carouge, D., Gensburger, C., Anglard, P., Burgun, C., Dietrich, J. B., et al. (2006). Fluoxetine and cocaine induce the epigenetic factors MeCP2 and MBD1 in adult rat brain. Molecular Pharmacology, 70(2), 487–492. doi:10.1124/mol.106.022301.
Chahrour, M., Jung, S. Y., Shaw, C., Zhou, X., Wong, S. T., Qin, J., et al. (2008). MeCP2, a key contributor to neurological disease, activates and represses transcription. Science, 320(5880), 1224–1229. doi:10.1126/science.1153252.
Chen, T. F., Huang, R. F., Lin, S. E., Lu, J. F., Tang, M. C., & Chiu, M. J. (2010a). Folic Acid potentiates the effect of memantine on spatial learning and neuronal protection in an Alzheimer’s disease transgenic model. Journal of Alzheimer’s Disease, 20(2), 607–615. doi:10.3233/JAD-2010-1396.
Chen, G., Zou, X., Watanabe, H., van Deursen, J. M., & Shen, J. (2010b). CREB binding protein is required for both short-term and long-term memory formation. Journal of Neuroscience, 30(39), 13066–13077. doi:10.1523/JNEUROSCI.2378-10.2010.
Colby, C. R., Whisler, K., Steffen, C., Nestler, E. J., & Self, D. W. (2003). Striatal cell type-specific overexpression of DeltaFosB enhances incentive for cocaine. Journal of Neuroscience, 23(6), 2488–2493.
Colussi, C., Mozzetta, C., Gurtner, A., Illi, B., Rosati, J., Straino, S., et al. (2008). HDAC2 blockade by nitric oxide and histone deacetylase inhibitors reveals a common target in Duchenne muscular dystrophy treatment. Proceedings of the National Academy of Sciences, 105(49), 19183–19187. doi:10.1073/pnas.0805514105.
Copray, S., Huynh, J. L., Sher, F., Casaccia-Bonnefil, P., & Boddeke, E. (2009). Epigenetic mechanisms facilitating oligodendrocyte development, maturation, and aging. Glia, 57(15), 1579–1587. doi:10.1002/glia.20881.
Deng, J. V., Rodriguiz, R. M., Hutchinson, A. N., Kim, I. H., Wetsel, W. C., & West, A. E. (2010). MeCP2 in the nucleus accumbens contributes to neural and behavioral responses to psychostimulants. Nature Neuroscience, 13(9), 1128–1136. doi:10.1038/nn.2614.
Dhalluin, C., Carlson, J. E., Zeng, L., He, C., Aggarwal, A. K., & Zhou, M. M. (1999). Structure and ligand of a histone acetyltransferase bromodomain. Nature, 399(6735), 491–496. doi:10.1038/20974.
Feng, J., Zhou, Y., Campbell, S. L., Le, T., Li, E., Sweatt, J. D., et al. (2010). Dnmt1 and Dnmt3a maintain DNA methylation and regulate synaptic function in adult forebrain neurons. Nature Neuroscience, 13(4), 423–430. doi:10.1038/nn.2514.
Fischer, A., Sananbenesi, F., Mungenast, A., & Tsai, L. H. (2010). Targeting the correct HDAC(s) to treat cognitive disorders. Trends in Pharmacological Sciences, 31(12), 605–617. doi:10.1016/j.tips.2010.09.003.
Fischer, A., Sananbenesi, F., Wang, X., Dobbin, M., & Tsai, L. H. (2007). Recovery of learning and memory is associated with chromatin remodelling. Nature, 447(7141), 178–182. doi:10.1038/nature05772.
Fontan-Lozano, A., Romero-Granados, R., Troncoso, J., Munera, A., Delgado-Garcia, J. M., & Carrion, A. M. (2008). Histone deacetylase inhibitors improve learning consolidation in young and in KA-induced-neurodegeneration and SAMP-8-mutant mice. Molecular and Cellular Neuroscience, 39(2), 193–201. doi:10.1016/j.mcn.2008.06.009.
Fontan-Lozano, A., Suarez-Pereira, I., Horrillo, A., del-Pozo-Martin, Y., Hmadcha, A., & Carrion, A. M. (2010). Histone H1 poly[ADP]-ribosylation regulates the chromatin alterations required for learning consolidation. Journal of Neuroscience, 30(40), 13305–13313. doi:10.1523/JNEUROSCI.3010-10.2010.
Francis, Y. I., Fa, M., Ashraf, H., Zhang, H., Staniszewski, A., Latchman, D. S., et al. (2009). Dysregulation of histone acetylation in the APP/PS1 mouse model of Alzheimer’s disease. Journal of Alzheimer’s Disease, 18(1), 131–139. doi:10.3233/JAD-2009-1134.
Fuso, A., Seminara, L., Cavallaro, R. A., D’Anselmi, F., & Scarpa, S. (2005). S-adenosylmethionine/homocysteine cycle alterations modify DNA methylation status with consequent deregulation of PS1 and BACE and beta-amyloid production. Molecular and Cellular Neuroscience, 28(1), 195–204. doi:10.1016/j.mcn.2004.09.007.
Goldberg, A. D., Allis, C. D., & Bernstein, E. (2007). Epigenetics: A landscape takes shape. Cell, 128(4), 635–638. doi:10.1016/j.cell.2007.02.006.
Govindarajan, N., Agis-Balboa, R. C., Walter, J., Sananbenesi, F., & Fischer, A. (2011). Sodium butyrate improves memory function in an Alzheimer’s disease mouse model when administered at an advanced stage of disease progression. Journal of Alzheimer’s Disease, 26(1), 187–197. doi:10.3233/JAD-2011-110080.
Graff, J., Kim, D., Dobbin, M. M., & Tsai, L. H. (2011). Epigenetic regulation of gene expression in physiological and pathological brain processes. Physiological Reviews, 91(2), 603–649. doi:10.1152/physrev.00012.2010.
Graff, J., & Mansuy, I. M. (2009). Epigenetic dysregulation in cognitive disorders. European Journal of Neuroscience, 30(1), 1–8. doi:10.1111/j.1460-9568.2009.06787.x.
Graff, J., Rei, D., Guan, J. S., Wang, W. Y., Seo, J., Hennig, K. M., et al. (2012). An epigenetic blockade of cognitive functions in the neurodegenerating brain. Nature, 483(7388), 222–226. doi:10.1038/nature10849.
Graham, D. L., Edwards, S., Bachtell, R. K., DiLeone, R. J., Rios, M., & Self, D. W. (2007). Dynamic BDNF activity in nucleus accumbens with cocaine use increases self-administration and relapse. Nature Neuroscience, 10(8), 1029–1037. doi:10.1038/nn1929.
Greer, E. L., & Shi, Y. (2012). Histone methylation: A dynamic mark in health, disease and inheritance. Nature Reviews Genetics, 13(5), 343–357. doi:10.1038/nrg3173.
Gregoretti, I. V., Lee, Y. M., & Goodson, H. V. (2004). Molecular evolution of the histone deacetylase family: Functional implications of phylogenetic analysis. Journal of Molecular Biology, 338(1), 17–31. doi:10.1016/j.jmb.2004.02.006.
Guan, Z., Giustetto, M., Lomvardas, S., Kim, J. H., Miniaci, M. C., Schwartz, J. H., et al. (2002). Integration of long-term-memory-related synaptic plasticity involves bidirectional regulation of gene expression and chromatin structure. Cell, 111(4), 483–493.
Guan, J. S., Haggarty, S. J., Giacometti, E., Dannenberg, J. H., Joseph, N., Gao, J., et al. (2009). HDAC2 negatively regulates memory formation and synaptic plasticity. Nature, 459(7243), 55–60. doi:10.1038/nature07925.
Gupta, S., Kim, S. Y., Artis, S., Molfese, D. L., Schumacher, A., Sweatt, J. D., et al. (2010). Histone methylation regulates memory formation. Journal of Neuroscience, 30(10), 3589–3599. doi:10.1523/JNEUROSCI.3732-09.2010.
Gupta-Agarwal, S., Franklin, A. V., Deramus, T., Wheelock, M., Davis, R. L., McMahon, L. L., et al. (2012). G9a/GLP histone lysine dimethyltransferase complex activity in the hippocampus and the entorhinal cortex is required for gene activation and silencing during memory consolidation. Journal of Neuroscience, 32(16), 5440–5453. doi:10.1523/JNEUROSCI.0147-12.2012.
Haberland, M., Montgomery, R. L., & Olson, E. N. (2009). The many roles of histone deacetylases in development and physiology: Implications for disease and therapy. Nature Reviews Genetics, 10(1), 32–42. doi:10.1038/nrg2485.
Haggarty, S. J., & Tsai, L. H. (2011). Probing the role of HDACs and mechanisms of chromatin-mediated neuroplasticity. Neurobiology of Learning and Memory, 96(1), 41–52. doi:10.1016/j.nlm.2011.04.009.
Hara, M. R., Agrawal, N., Kim, S. F., Cascio, M. B., Fujimuro, M., Ozeki, Y., et al. (2005). S-nitrosylated GAPDH initiates apoptotic cell death by nuclear translocation following Siah1 binding. Nature Cell Biology, 7(7), 665–674. doi:10.1038/ncb1268.
Hargreaves, D. C., Horng, T., & Medzhitov, R. (2009). Control of inducible gene expression by signal-dependent transcriptional elongation. Cell, 138(1), 129–145. doi:10.1016/j.cell.2009.05.047.
Hess, D. T., Matsumoto, A., Kim, S. O., Marshall, H. E., & Stamler, J. S. (2005). Protein S-nitrosylation: Purview and parameters. Nature Reviews Molecular Cell Biology, 6(2), 150–166. doi:10.1038/nrm1569.
Holtzman, D. M., Goate, A., Kelly, J., & Sperling, R. (2011). Mapping the road forward in Alzheimer’s disease. Science Translational Medicine, 3(114), 114ps148. doi:10.1126/scitranslmed.3003529.
Host, L., Dietrich, J. B., Carouge, D., Aunis, D., & Zwiller, J. (2011). Cocaine self-administration alters the expression of chromatin-remodelling proteins; modulation by histone deacetylase inhibition. Journal of Psychopharmacology, 25(2), 222–229. doi:10.1177/0269881109348173.
Huang, Y., & Mucke, L. (2012). Alzheimer mechanisms and therapeutic strategies. Cell, 148(6), 1204–1222. doi:10.1016/j.cell.2012.02.040.
Im, H. I., Hollander, J. A., Bali, P., & Kenny, P. J. (2010). MeCP2 controls BDNF expression and cocaine intake through homeostatic interactions with microRNA-212. Nature Neuroscience, 13(9), 1120–1127. doi:10.1038/nn.2615.
Jaenisch, R., & Bird, A. (2003). Epigenetic regulation of gene expression: How the genome integrates intrinsic and environmental signals. Nature Genetics, 33(Suppl), 245–254. doi:10.1038/ng1089.
Jenuwein, T., & Allis, C. D. (2001). Translating the histone code. Science, 293(5532), 1074–1080. doi:10.1126/science.1063127.
Jones, P. L., Veenstra, G. J., Wade, P. A., Vermaak, D., Kass, S. U., Landsberger, N., et al. (1998). Methylated DNA and MeCP2 recruit histone deacetylase to repress transcription. Nature Genetics, 19(2), 187–191. doi:10.1038/561.
Kalkhoven, E. (2004). CBP and p300: HATs for different occasions. Biochemical Pharmacology, 68(6), 1145–1155. doi:10.1016/j.bcp.2004.03.045.
Kazantsev, A. G., & Thompson, L. M. (2008). Therapeutic application of histone deacetylase inhibitors for central nervous system disorders. Nature Reviews Drug Discovery, 7(10), 854–868. doi:10.1038/nrd2681.
Kelz, M. B., Chen, J., Carlezon, W. A, Jr, Whisler, K., Gilden, L., Beckmann, A. M., et al. (1999). Expression of the transcription factor deltaFosB in the brain controls sensitivity to cocaine. Nature, 401(6750), 272–276. doi:10.1038/45790.
Kim, W. Y., Kim, S., & Kim, J. H. (2008). Chronic microinjection of valproic acid into the nucleus accumbens attenuates amphetamine-induced locomotor activity. Neuroscience Letters, 432(1), 54–57. doi:10.1016/j.neulet.2007.12.005.
Kornhauser, J. M., Cowan, C. W., Shaywitz, A. J., Dolmetsch, R. E., Griffith, E. C., Hu, L. S., et al. (2002). CREB transcriptional activity in neurons is regulated by multiple, calcium-specific phosphorylation events. Neuron, 34(2), 221–233.
Korzus, E., Rosenfeld, M. G., & Mayford, M. (2004). CBP histone acetyltransferase activity is a critical component of memory consolidation. Neuron, 42(6), 961–972. doi:10.1016/j.neuron.2004.06.002.
Koshibu, K., Graff, J., Beullens, M., Heitz, F. D., Berchtold, D., Russig, H., et al. (2009). Protein phosphatase 1 regulates the histone code for long-term memory. Journal of Neuroscience, 29(41), 13079–13089. doi:10.1523/JNEUROSCI.3610-09.2009.
Kumar, A., Choi, K. H., Renthal, W., Tsankova, N. M., Theobald, D. E., Truong, H. T., et al. (2005). Chromatin remodeling is a key mechanism underlying cocaine-induced plasticity in striatum. Neuron, 48(2), 303–314. doi:10.1016/j.neuron.2005.09.023.
Lamprecht, R., Hazvi, S., & Dudai, Y. (1997). cAMP response element-binding protein in the amygdala is required for long- but not short-term conditioned taste aversion memory. Journal of Neuroscience, 17(21), 8443–8450.
LaPlant, Q., Vialou, V., Covington, H. E, 3rd, Dumitriu, D., Feng, J., Warren, B. L., et al. (2010). Dnmt3a regulates emotional behavior and spine plasticity in the nucleus accumbens. Nature Neuroscience, 13(9), 1137–1143. doi:10.1038/nn.2619.
Lattal, K. M., Barrett, R. M., & Wood, M. A. (2007). Systemic or intrahippocampal delivery of histone deacetylase inhibitors facilitates fear extinction. Behavioral Neuroscience, 121(5), 1125–1131. doi:10.1037/0735-7044.121.5.1125.
Lee, Y. S., & Silva, A. J. (2009). The molecular and cellular biology of enhanced cognition. Nature Reviews Neuroscience, 10(2), 126–140. doi:10.1038/nrn2572.
Lee, K. K., & Workman, J. L. (2007). Histone acetyltransferase complexes: One size doesn’t fit all. Nature Reviews Molecular Cell Biology, 8(4), 284–295. doi:10.1038/nrm2145.
Lesburgueres, E., Gobbo, O. L., Alaux-Cantin, S., Hambucken, A., Trifilieff, P., & Bontempi, B. (2011). Early tagging of cortical networks is required for the formation of enduring associative memory. Science, 331(6019), 924–928. doi:10.1126/science.1196164.
Levenson, J. M., O’Riordan, K. J., Brown, K. D., Trinh, M. A., Molfese, D. L., & Sweatt, J. D. (2004). Regulation of histone acetylation during memory formation in the hippocampus. Journal of Biological Chemistry, 279(39), 40545–40559. doi:10.1074/jbc.M402229200.
Levine, A. A., Guan, Z., Barco, A., Xu, S., Kandel, E. R., & Schwartz, J. H. (2005). CREB-binding protein controls response to cocaine by acetylating histones at the fosB promoter in the mouse striatum. Proceedings of the National Academy of Sciences of the United States of America, 102(52), 19186–19191. doi:10.1073/pnas.0509735102.
Liu, X., Wang, L., Zhao, K., Thompson, P. R., Hwang, Y., Marmorstein, R., et al. (2008). The structural basis of protein acetylation by the p300/CBP transcriptional coactivator. Nature, 451(7180), 846–850. doi:10.1038/nature06546.
Lopez-Atalaya, J. P., Gervasini, C., Mottadelli, F., Spena, S., Piccione, M., Scarano, G., et al. (2012). Histone acetylation deficits in lymphoblastoid cell lines from patients with Rubinstein–Taybi syndrome. Journal of Medical Genetics, 49(1), 66–74. doi:10.1136/jmedgenet-2011-100354.
Lu, T., Pan, Y., Kao, S. Y., Li, C., Kohane, I., Chan, J., et al. (2004). Gene regulation and DNA damage in the ageing human brain. Nature, 429(6994), 883–891. doi:10.1038/nature02661.
Lubin, F. D., Roth, T. L., & Sweatt, J. D. (2008). Epigenetic regulation of BDNF gene transcription in the consolidation of fear memory. Journal of Neuroscience, 28(42), 10576–10586. doi:10.1523/JNEUROSCI.1786-08.2008.
Lubin, F. D., & Sweatt, J. D. (2007). The IkappaB kinase regulates chromatin structure during reconsolidation of conditioned fear memories. Neuron, 55(6), 942–957. doi:10.1016/j.neuron.2007.07.039.
Ma, D. K., Jang, M. H., Guo, J. U., Kitabatake, Y., Chang, M. L., Pow-Anpongkul, N., et al. (2009). Neuronal activity-induced Gadd45b promotes epigenetic DNA demethylation and adult neurogenesis. Science, 323(5917), 1074–1077. doi:10.1126/science.1166859.
MacDonald, J. L., & Roskams, A. J. (2008). Histone deacetylases 1 and 2 are expressed at distinct stages of neuro-glial development. Developmental Dynamics, 237(8), 2256–2267. doi:10.1002/dvdy.21626.
Maddox, S. A., & Schafe, G. E. (2011). Epigenetic alterations in the lateral amygdala are required for reconsolidation of a Pavlovian fear memory. Learning & Memory, 18(9), 579–593. doi:10.1101/lm.2243411.
Maze, I., Covington, H. E, 3rd, Dietz, D. M., LaPlant, Q., Renthal, W., Russo, S. J., et al. (2010). Essential role of the histone methyltransferase G9a in cocaine-induced plasticity. Science, 327(5962), 213–216. doi:10.1126/science.1179438.
Maze, I., & Nestler, E. J. (2011). The epigenetic landscape of addiction. Annals of the New York Academy of Sciences, 1216, 99–113. doi:10.1111/j.1749-6632.2010.05893.x.
McQuown, S. C., Barrett, R. M., Matheos, D. P., Post, R. J., Rogge, G. A., Alenghat, T., et al. (2011). HDAC3 is a critical negative regulator of long-term memory formation. Journal of Neuroscience, 31(2), 764–774. doi:10.1523/JNEUROSCI.5052-10.2011.
McQuown, S. C., & Wood, M. A. (2010). Epigenetic regulation in substance use disorders. Current Psychiatry Reports, 12(2), 145–153. doi:10.1007/s11920-010-0099-5.
Michan, S., & Sinclair, D. (2007). Sirtuins in mammals: Insights into their biological function. Biochemical Journal, 404(1), 1–13. doi:10.1042/BJ20070140.
Miller, C. A., Gavin, C. F., White, J. A., Parrish, R. R., Honasoge, A., Yancey, C. R., et al. (2010). Cortical DNA methylation maintains remote memory. Nature Neuroscience, 13(6), 664–666. doi:10.1038/nn.2560.
Miller, C. A., & Sweatt, J. D. (2007). Covalent modification of DNA regulates memory formation. Neuron, 53(6), 857–869. doi:10.1016/j.neuron.2007.02.022.
Monsey, M. S., Ota, K. T., Akingbade, I. F., Hong, E. S., & Schafe, G. E. (2011). Epigenetic alterations are critical for fear memory consolidation and synaptic plasticity in the lateral amygdala. PLoS ONE, 6(5), e19958. doi:10.1371/journal.pone.0019958.
Moretti, P., Levenson, J. M., Battaglia, F., Atkinson, R., Teague, R., Antalffy, B., et al. (2006). Learning and memory and synaptic plasticity are impaired in a mouse model of Rett syndrome. Journal of Neuroscience, 26(1), 319–327. doi:10.1523/JNEUROSCI.2623-05.2006.
Morris, M. J., Karra, A. S., & Monteggia, L. M. (2010). Histone deacetylases govern cellular mechanisms underlying behavioral and synaptic plasticity in the developing and adult brain. Behavioural Pharmacology, 21(5–6), 409–419. doi:10.1097/FBP.0b013e32833c20c0.
Nott, A., Watson, P. M., Robinson, J. D., Crepaldi, L., & Riccio, A. (2008). S-Nitrosylation of histone deacetylase 2 induces chromatin remodelling in neurons. Nature, 455(7211), 411–415. doi:10.1038/nature07238.
Oliveira, A. M., Wood, M. A., McDonough, C. B., & Abel, T. (2007). Transgenic mice expressing an inhibitory truncated form of p300 exhibit long-term memory deficits. Learning & Memory, 14(9), 564–572. doi:10.1101/lm.656907.
Peleg, S., Sananbenesi, F., Zovoilis, A., Burkhardt, S., Bahari-Javan, S., Agis-Balboa, R. C., et al. (2010). Altered histone acetylation is associated with age-dependent memory impairment in mice. Science, 328(5979), 753–756. doi:10.1126/science.1186088.
Peters, A. H., & Schubeler, D. (2005). Methylation of histones: Playing memory with DNA. Current Opinion in Cell Biology, 17(2), 230–238. doi:10.1016/j.ceb.2005.02.006.
Petrij, F., Giles, R. H., Dauwerse, H. G., Saris, J. J., Hennekam, R. C., Masuno, M., et al. (1995). Rubinstein–Taybi syndrome caused by mutations in the transcriptional co-activator CBP. Nature, 376(6538), 348–351. doi:10.1038/376348a0.
Portela, A., & Esteller, M. (2010). Epigenetic modifications and human disease. Nature Biotechnology, 28(10), 1057–1068. doi:10.1038/nbt.1685.
Putignano, E., Lonetti, G., Cancedda, L., Ratto, G., Costa, M., Maffei, L., et al. (2007). Developmental downregulation of histone posttranslational modifications regulates visual cortical plasticity. Neuron, 53(5), 747–759. doi:10.1016/j.neuron.2007.02.007.
Ramos, Y. F., Hestand, M. S., Verlaan, M., Krabbendam, E., Ariyurek, Y., van Galen, M., et al. (2010). Genome-wide assessment of differential roles for p300 and CBP in transcription regulation. Nucleic Acids Research, 38(16), 5396–5408. doi:10.1093/nar/gkq184.
Renthal, W., Kumar, A., Xiao, G., Wilkinson, M., Covington, H. E, 3rd, Maze, I., et al. (2009). Genome-wide analysis of chromatin regulation by cocaine reveals a role for sirtuins. Neuron, 62(3), 335–348. doi:10.1016/j.neuron.2009.03.026.
Renthal, W., Maze, I., Krishnan, V., Covington, H. E, 3rd, Xiao, G., Kumar, A., et al. (2007). Histone deacetylase 5 epigenetically controls behavioral adaptations to chronic emotional stimuli. Neuron, 56(3), 517–529. doi:10.1016/j.neuron.2007.09.032.
Renthal, W., & Nestler, E. J. (2008). Epigenetic mechanisms in drug addiction. Trends in Molecular Medicine, 14(8), 341–350. doi:10.1016/j.molmed.2008.06.004.
Riccio, A. (2010). Dynamic epigenetic regulation in neurons: Enzymes, stimuli and signaling pathways. Nature Neuroscience, 13(11), 1330–1337. doi:10.1038/nn.2671.
Rice, J. C., Briggs, S. D., Ueberheide, B., Barber, C. M., Shabanowitz, J., Hunt, D. F., et al. (2003). Histone methyltransferases direct different degrees of methylation to define distinct chromatin domains. Molecular Cell, 12(6), 1591–1598.
Ricobaraza, A., Cuadrado-Tejedor, M., Perez-Mediavilla, A., Frechilla, D., Del Rio, J., & Garcia-Osta, A. (2009). Phenylbutyrate ameliorates cognitive deficit and reduces tau pathology in an Alzheimer’s disease mouse model. Neuropsychopharmacology, 34(7), 1721–1732. doi:10.1038/npp.2008.229.
Roelfsema, J. H., & Peters, D. J. (2007). Rubinstein–Taybi syndrome: Clinical and molecular overview. Expert Reviews in Molecular Medicine, 9(23), 1–16. doi:10.1017/S1462399407000415.
Roelfsema, J. H., White, S. J., Ariyurek, Y., Bartholdi, D., Niedrist, D., Papadia, F., et al. (2005). Genetic heterogeneity in Rubinstein–Taybi syndrome: Mutations in both the CBP and EP300 genes cause disease. American Journal of Human Genetics, 76(4), 572–580. doi:10.1086/429130.
Romieu, P., Host, L., Gobaille, S., Sandner, G., Aunis, D., & Zwiller, J. (2008). Histone deacetylase inhibitors decrease cocaine but not sucrose self-administration in rats. Journal of Neuroscience, 28(38), 9342–9348. doi:10.1523/JNEUROSCI.0379-08.2008.
Roth, S. Y., Denu, J. M., & Allis, C. D. (2001). Histone acetyltransferases. Annual Review of Biochemistry, 70, 81–120. doi:10.1146/annurev.biochem.70.1.81.
Saura, C. A., Choi, S. Y., Beglopoulos, V., Malkani, S., Zhang, D., Shankaranarayana Rao, B. S., et al. (2004). Loss of presenilin function causes impairments of memory and synaptic plasticity followed by age-dependent neurodegeneration. Neuron, 42(1), 23–36.
Schroeder, F. A., Penta, K. L., Matevossian, A., Jones, S. R., Konradi, C., Tapper, A. R., et al. (2008). Drug-induced activation of dopamine D(1) receptor signaling and inhibition of class I/II histone deacetylase induce chromatin remodeling in reward circuitry and modulate cocaine-related behaviors. Neuropsychopharmacology, 33(12), 2981–2992. doi:10.1038/npp.2008.15.
Sen, N., Hara, M. R., Kornberg, M. D., Cascio, M. B., Bae, B. I., Shahani, N., et al. (2008). Nitric oxide-induced nuclear GAPDH activates p300/CBP and mediates apoptosis. Nature Cell Biology, 10(7), 866–873. doi:10.1038/ncb1747.
Sen, N., & Snyder, S. H. (2011). Neurotrophin-mediated degradation of histone methyltransferase by S-nitrosylation cascade regulates neuronal differentiation. Proceedings of the National Academy of Sciences of the United States of America, 108(50), 20178–20183. doi:10.1073/pnas.1117820108.
Shahani, N., & Sawa, A. (2011). Nitric oxide signaling and nitrosative stress in neurons: Role for S-nitrosylation. Antioxidants & Redox Signaling, 14(8), 1493–1504. doi:10.1089/ars.2010.3580.
Shaywitz, A. J., & Greenberg, M. E. (1999). CREB: A stimulus-induced transcription factor activated by a diverse array of extracellular signals. Annual Review of Biochemistry, 68, 821–861. doi:10.1146/annurev.biochem.68.1.821.
Sterner, D. E., & Berger, S. L. (2000). Acetylation of histones and transcription-related factors. Microbiology and Molecular Biology Reviews, 64(2), 435–459.
Stevens, C. F. (1994). CREB and memory consolidation. Neuron, 13(4), 769–770.
Sultan, F. A., & Day, J. J. (2011). Epigenetic mechanisms in memory and synaptic function. Epigenomics, 3(2), 157–181. doi:10.2217/epi.11.6.
Suzuki, M. M., & Bird, A. (2008). DNA methylation landscapes: Provocative insights from epigenomics. Nature Reviews Genetics, 9(6), 465–476. doi:10.1038/nrg2341.
Tang, B., Chang, W. L., Lanigan, C. M., Dean, B., Sutcliffe, J. G., & Thomas, E. A. (2009). Normal human aging and early-stage schizophrenia share common molecular profiles. Aging Cell, 8(3), 339–342. doi:10.1111/j.1474-9726.2009.00468.x.
Tricoire, L., & Vitalis, T. (2012). Neuronal nitric oxide synthase expressing neurons: A journey from birth to neuronal circuits. Front Neural Circuits, 6, 82. doi:10.3389/fncir.2012.00082.
Trievel, R. C. (2004). Structure and function of histone methyltransferases. Critical Reviews in Eukaryotic Gene Expression, 14(3), 147–169.
Vashishtha, M., Ng, C. W., Yildirim, F., Gipson, T. A., Kratter, I. H., Bodai, L., et al. (2013). Targeting H3K4 trimethylation in Huntington disease. Proceedings of the National Academy of Sciences of the United States of America, 110(32), E3027–3036. doi:10.1073/pnas.1311323110.
Vecsey, C. G., Hawk, J. D., Lattal, K. M., Stein, J. M., Fabian, S. A., Attner, M. A., et al. (2007). Histone deacetylase inhibitors enhance memory and synaptic plasticity via CREB:CBP-dependent transcriptional activation. Journal of Neuroscience, 27(23), 6128–6140. doi:10.1523/JNEUROSCI.0296-07.2007.
Walsh, D. M., & Selkoe, D. J. (2004). Deciphering the molecular basis of memory failure in Alzheimer’s disease. Neuron, 44(1), 181–193. doi:10.1016/j.neuron.2004.09.010.
Walton, M. R., & Dragunow, I. (2000). Is CREB a key to neuronal survival? Trends in Neurosciences, 23(2), 48–53.
Wang, W. H., Cheng, L. C., Pan, F. Y., Xue, B., Wang, D. Y., Chen, Z., et al. (2011). Intracellular trafficking of histone deacetylase 4 regulates long-term memory formation. The Anatomical Record (Hoboken), 294(6), 1025–1034. doi:10.1002/ar.21389.
Wang, C. M., Tsai, S. N., Yew, T. W., Kwan, Y. W., & Ngai, S. M. (2010). Identification of histone methylation multiplicities patterns in the brain of senescence-accelerated prone mouse 8. Biogerontology, 11(1), 87–102. doi:10.1007/s10522-009-9231-5.
Wood, M. A., Kaplan, M. P., Park, A., Blanchard, E. J., Oliveira, A. M., Lombardi, T. L., et al. (2005). Transgenic mice expressing a truncated form of CREB-binding protein (CBP) exhibit deficits in hippocampal synaptic plasticity and memory storage. Learning & Memory, 12(2), 111–119. doi:10.1101/lm.86605.
Wu, S. C., & Zhang, Y. (2010). Active DNA demethylation: Many roads lead to Rome. Nature Reviews Molecular Cell Biology, 11(9), 607–620. doi:10.1038/nrm2950.
Xu, R., Serritella, A. V., Sen, T., Farook, J. M., Sedlak, T. W., Baraban, J., et al. (2013). Behavioral effects of cocaine mediated by nitric oxide-GAPDH transcriptional signaling. Neuron, 78(4), 623–630. doi:10.1016/j.neuron.2013.03.021.
Yang, X. J. (2004). Lysine acetylation and the bromodomain: A new partnership for signaling. BioEssays, 26(10), 1076–1087. doi:10.1002/bies.20104.
Yang, X. J., & Seto, E. (2008). The Rpd3/Hda1 family of lysine deacetylases: From bacteria and yeast to mice and men. Nature Reviews Molecular Cell Biology, 9(3), 206–218. doi:10.1038/nrm2346.
Yankner, B. A., Lu, T., & Loerch, P. (2008). The aging brain. Annual Review of Pathology: Mechanisms of Disease, 3, 41–66. doi:10.1146/annurev.pathmechdis.2.010506.092044.
Yeh, S. H., Lin, C. H., & Gean, P. W. (2004). Acetylation of nuclear factor-kappaB in rat amygdala improves long-term but not short-term retention of fear memory. Molecular Pharmacology, 65(5), 1286–1292. doi:10.1124/mol.65.5.1286.
Zachariou, V., Bolanos, C. A., Selley, D. E., Theobald, D., Cassidy, M. P., Kelz, M. B., et al. (2006). An essential role for DeltaFosB in the nucleus accumbens in morphine action. Nature Neuroscience, 9(2), 205–211. doi:10.1038/nn1636.
Zhang, Y., & Reinberg, D. (2001). Transcription regulation by histone methylation: Interplay between different covalent modifications of the core histone tails. Genes & Development, 15(18), 2343–2360. doi:10.1101/gad.927301.
Zhou, L., & Zhu, D. Y. (2009). Neuronal nitric oxide synthase: Structure, subcellular localization, regulation, and clinical implications. Nitric Oxide, 20(4), 223–230. doi:10.1016/j.niox.2009.03.001.
Acknowledgments
We express our apologies to colleagues whose work could not be included in this review. N.S is supported by a generous startup funding provided by Georgia Regents University.
Conflict of interest
I would like to declare that I do not have any conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sen, N. Epigenetic Regulation of Memory by Acetylation and Methylation of Chromatin: Implications in Neurological Disorders, Aging, and Addiction. Neuromol Med 17, 97–110 (2015). https://doi.org/10.1007/s12017-014-8306-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12017-014-8306-x