Abstract
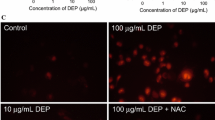
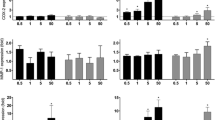
Epidemiological studies suggest that an increase of diesel exhaust particles (DEP) in ambient air corresponds to an increase in hospital-recorded myocardial infarctions within 48 h after exposure. Among the many theories to explain this data are endothelial dysfunction and translocation of DEP into vasculature. The mechanisms for such DEP-induced vascular permeability remain unknown. One of the major mechanisms underlying the effects of DEP is suggested to be oxidative stress. Experiments have shown that DEP induce the generation of reactive oxygen species (ROS), such as superoxide anion and H2O2 in the HUVEC tube cells. Transcription factor Nrf2 is translocated to the cell nucleus, where it activates transcription of the antioxidative enzyme HO-1 and sequentially induces the release of vascular permeability factor VEGF-A. Furthermore, a recent study shows that DEP-induced intracellular ROS may cause the release of pro-inflammatory TNF-α and IL-6, which may induce endothelial permeability as well by promoting VEGF-A secretion independently of HO-1 activation. These results demonstrated that the adherens junction molecule, VE-cadherin, becomes redistributed from the membrane at cell–cell borders to the cytoplasm in response to DEP, separating the plasma membranes of adjacent cells. DEP were occasionally found in endothelial cell cytoplasm and in tube lumen. In addition, the induced ROS is cytotoxic to the endothelial tube-like HUVEC. Acute DEP exposure stimulates ATP depletion, followed by depolarization of their actin cytoskeleton, which sequentially inhibits PI3K/Akt activity and induces endothelial apoptosis. Nevertheless, high-dose DEP augments tube cell apoptosis up to 70 % but disrupts the p53 negative regulator Mdm2. In summary, exposure to DEP affects parameters influencing vasculature permeability and viability, i.e., oxidative stress and its upregulated antioxidative and pro-inflammatory responses, which sequentially induce vascular permeability factor, VEGF-A release and disrupt cell–cell junction integrity. While exposure to a low dose of DEP actin triggers cytoskeleton depolarization, reduces PI3K/Akt activity, and induces a p53/Mdm2 feedback loop, a high dose causes apoptosis by depleting Mdm2. Addition of ROS scavenger N-acetyl cysteine suppresses DEP-induced oxidative stress efficiently and reduces subsequent damages by increasing endogenous glutathione.

Similar content being viewed by others
References
IARC. (2012). Diesel engine exhaust carcinogenic. Central European Journal of Public Health, 20, 120–138.
Ghio, A. J., Sobus, J. R., Pleil, J. D., & Madden, M. C. (2012). Controlled human exposures to diesel exhaust. Swiss Medical Weekly, 142, w13597.
Brook, R. D. (2008). Cardiovascular effects of air pollution. Clinical Science (London), 115, 175–187.
Pope, C. A, I. I. I., & Dockery, D. W. (2006). Health effects of fine particulate air pollution: Lines that connect. Journal of the Air & Waste Management Association (1995), 56, 709–742.
Sydbom, A., Blomberg, A., Parnia, S., Stenfors, N., Sandstrom, T., & Dahlen, S. E. (2001). Health effects of diesel exhaust emissions. European Respiratory Journal, 17, 733–746.
Krivoshto, I. N., Richards, J. R., Albertson, T. E., & Derlet, R. W. (2008). The toxicity of diesel exhaust: Implications for primary care. The Journal of the American Board of Family Medicine, 21, 55–62.
Mamessier, E., Nieves, A., Vervloet, D., & Magnan, A. (2006). Diesel exhaust particles enhance T-cell activation in severe asthmatics. Allergy, 61, 581–588.
Ishinishi, N., Kuwabara, N., Nagase, S., Suzuki, T., Ishiwata, S., & Kohno, T. (1986). Long-term inhalation studies on effects of exhaust from heavy and light duty diesel engines on F344 rats. Developments in Toxicology and Environmental Science, 13, 329–348.
McClellan, R. O. (1987). Health effects of exposure to diesel exhaust particles. Annual Review of Pharmacology and Toxicology, 27, 279–300.
Kumagai, Y., Arimoto, T., Shinyashiki, M., Shimojo, N., Nakai, Y., Yoshikawa, T., & Sagai, M. (1997). Generation of reactive oxygen species during interaction of diesel exhaust particle components with NADPH-cytochrome P450 reductase and involvement of the bioactivation in the DNA damage. Free Radical Biology and Medicine, 22, 479–487.
Wichmann, H. E. (2007). Diesel exhaust particles. Inhalation Toxicology, 19(Suppl 1), 241–244.
Sun, Y., Bochmann, F., Nold, A., & Mattenklott, M. (2014). Diesel exhaust exposure and the risk of lung cancer—A review of the epidemiological evidence. International Journal of Environmental Research and Public Health, 11, 1312–1340.
Hodan, W.M., Barnard, W. R. (2004). Evaluating the contribution of PM2.5 precursor gases and re-entrained road emissions to mobile source PM2.5 particulate matter emissions. MACTEC Federal Programs, Research Triangle Park, NC.
Monn, C., Fuchs, A., Hogger, D., Junker, M., Kogelschatz, D., Roth, N., & Wanner, H. U. (1997). Particulate matter less than 10 microns (PM10) and fine particles less than 2.5 microns (PM2.5): Relationships between indoor, outdoor and personal concentrations. Science of the Total Environment, 208, 15–21.
Nordman, H., Keskinen, H., & Tuppurainen, M. (1985). Formaldehyde asthma—rare or overlooked? Journal of Allergy and Clinical Immunology, 75, 91–99.
Kerns, W. D., Pavkov, K. L., Donofrio, D. J., Gralla, E. J., & Swenberg, J. A. (1983). Carcinogenicity of formaldehyde in rats and mice after long-term inhalation exposure. Cancer Research, 43, 4382–4392.
Suwa, T., Hogg, J. C., Quinlan, K. B., Ohgami, A., Vincent, R., & van Eeden, S. F. (2002). Particulate air pollution induces progression of atherosclerosis. Journal of the American College of Cardiology, 39, 935–942.
Miller, M. R., Shaw, C. A., & Langrish, J. P. (2012). From particles to patients: Oxidative stress and the cardiovascular effects of air pollution. Future Cardiology, 8, 577–602.
Pope, C. A, 3rd. (2007). Mortality effects of longer term exposures to fine particulate air pollution: Review of recent epidemiological evidence. Inhalation Toxicology, 19(Suppl 1), 33–38.
Finkelstein, M. M., Verma, D. K., Sahai, D., & Stefov, E. (2004). Ischemic heart disease mortality among heavy equipment operators. American Journal of Industrial Medicine, 46, 16–22.
Mills, N. L., Tornqvist, H., Gonzalez, M. C., Vink, E., Robinson, S. D., Soderberg, S., et al. (2007). Ischemic and thrombotic effects of dilute diesel-exhaust inhalation in men with coronary heart disease. The New England Journal of Medicine, 357, 1075–1082.
Peters, A., von Klot, S., Heier, M., Trentinaglia, I., Hormann, A., Wichmann, H. E., & Lowel, H. (2004). Exposure to traffic and the onset of myocardial infarction. The New England Journal of Medicine, 351, 1721–1730.
Anselme, F., Loriot, S., Henry, J. P., Dionnet, F., Napoleoni, J. G., Thuillez, C., & Morin, J. P. (2007). Inhalation of diluted diesel engine emission impacts heart rate variability and arrhythmia occurrence in a rat model of chronic ischemic heart failure. Archives of Toxicology, 81, 299–307.
Wold, L. E., Simkhovich, B. Z., Kleinman, M. T., Nordlie, M. A., Dow, J. S., Sioutas, C., & Kloner, R. A. (2006). In vivo and in vitro models to test the hypothesis of particle-induced effects on cardiac function and arrhythmias. Cardiovascular Toxicology, 6, 69–78.
Mills, N. L., Tornqvist, H., Robinson, S. D., Gonzalez, M., Darnley, K., MacNee, W., et al. (2005). Diesel exhaust inhalation causes vascular dysfunction and impaired endogenous fibrinolysis. Circulation, 112, 3930–3936.
Baccarelli, A., Zanobetti, A., Martinelli, I., Grillo, P., Hou, L., Giacomini, S., et al. (2007). Effects of exposure to air pollution on blood coagulation. Journal of Thrombosis and Haemostasis, 5, 252–260.
Mates, J. M., Segura, J. A., Alonso, F. J., & Marquez, J. (2008). Intracellular redox status and oxidative stress: Implications for cell proliferation, apoptosis, and carcinogenesis. Archives of Toxicology, 82, 273–299.
Garza, K. M., Soto, K. F., & Murr, L. E. (2008). Cytotoxicity and reactive oxygen species generation from aggregated carbon and carbonaceous nanoparticulate materials. International Journal of Nanomedicine, 3, 83–94.
Matsunaga, T., Arakaki, M., Kamiya, T., Endo, S., El-Kabbani, O., & Hara, A. (2009). Involvement of an aldo-keto reductase (AKR1C3) in redox cycling of 9,10-phenanthrenequinone leading to apoptosis in human endothelial cells. Chemico-Biological Interactions, 181, 52–60.
Matsuo, M., Shimada, T., Uenishi, R., Sasaki, N., & Sagai, M. (2003). Diesel exhaust particle-induced cell death of cultured normal human bronchial epithelial cells. Biological and Pharmaceutical Bulletin, 26, 438–447.
Hawley, B., L’Orange, C., Olsen, D. B., Marchese, A. J., & Volckens, J. (2014). Oxidative stress and aromatic hydrocarbon response of human bronchial epithelial cells exposed to petro- or biodiesel exhaust treated with a diesel particulate filter. Toxicological Sciences, 141, 505–514.
Hiura, T. S., Kaszubowski, M. P., Li, N., & Nel, A. E. (1999). Chemicals in diesel exhaust particles generate reactive oxygen radicals and induce apoptosis in macrophages. The Journal of Immunology, 163, 5582–5591.
Hiura, T. S., Li, N., Kaplan, R., Horwitz, M., Seagrave, J. C., & Nel, A. E. (2000). The role of a mitochondrial pathway in the induction of apoptosis by chemicals extracted from diesel exhaust particles. The Journal of Immunology, 165, 2703–2711.
Li, N., Kim, S., Wang, M., Froines, J., Sioutas, C., & Nel, A. (2002). Use of a stratified oxidative stress model to study the biological effects of ambient concentrated and diesel exhaust particulate matter. Inhalation Toxicology, 14, 459–486.
Sagai, M., Saito, H., Ichinose, T., Kodama, M., & Mori, Y. (1993). Biological effects of diesel exhaust particles. I. In vitro production of superoxide and in vivo toxicity in mouse. Free Radical Biology and Medicine, 14, 37–47.
Moller, P., Jensen, D. M., Christophersen, D. V., Kermanizadeh, A., Jacobsen, N. R., Hemmingsen, J. G., et al. (2015). Measurement of oxidative damage to DNA in nanomaterial exposed cells and animals. Environmental and Molecular Mutagenesis, 56, 97–110.
Sugimoto, R., Kumagai, Y., Nakai, Y., & Ishii, T. (2005). 9,10-Phenanthraquinone in diesel exhaust particles downregulates Cu, Zn-SOD and HO-1 in human pulmonary epithelial cells: Intracellular iron scavenger 1,10-phenanthroline affords protection against apoptosis. Free Radical Biology and Medicine, 38, 388–395.
Takizawa, H., Ohtoshi, T., Kawasaki, S., Abe, S., Sugawara, I., Nakahara, K., et al. (2000). Diesel exhaust particles activate human bronchial epithelial cells to express inflammatory mediators in the airways: A review. Respirology (Carlton Vic), 5, 197–203.
Al-Humadi, N. H., Siegel, P. D., Lewis, D. M., Barger, M. W., Ma, J. Y., Weissman, D. N., & Ma, J. K. (2002). Alteration of intracellular cysteine and glutathione levels in alveolar macrophages and lymphocytes by diesel exhaust particle exposure. Environmental Health Perspectives, 110, 349–353.
Bai, Y., Suzuki, A. K., & Sagai, M. (2001). The cytotoxic effects of diesel exhaust particles on human pulmonary artery endothelial cells in vitro: Role of active oxygen species. Free Radical Biology and Medicine, 30, 555–562.
Park, S., Nam, H., Chung, N., Park, J. D., & Lim, Y. (2006). The role of iron in reactive oxygen species generation from diesel exhaust particles. Toxicology in Vitro, 20, 851–857.
Langrish, J. P., Unosson, J., Bosson, J., Barath, S., Muala, A., Blackwell, S., et al. (2013). Altered nitric oxide bioavailability contributes to diesel exhaust inhalation-induced cardiovascular dysfunction in man. Journal of the American Heart Association, 2, e004309.
Jiang, J. G., Chen, R. J., Xiao, B., Yang, S., Wang, J. N., Wang, Y., et al. (2007). Regulation of endothelial nitric-oxide synthase activity through phosphorylation in response to epoxyeicosatrienoic acids. Prostaglandins & Other Lipid Mediators, 82, 162–174.
Cherng, T. W., Paffett, M. L., Jackson-Weaver, O., Campen, M. J., Walker, B. R., & Kanagy, N. L. (2011). Mechanisms of diesel-induced endothelial nitric oxide synthase dysfunction in coronary arterioles. Environmental Health Perspectives, 119, 98–103.
Bonvallot, V., Baeza-Squiban, A., Baulig, A., Brulant, S., Boland, S., Muzeau, F., et al. (2001). Organic compounds from diesel exhaust particles elicit a proinflammatory response in human airway epithelial cells and induce cytochrome p450 1A1 expression. American Journal of Respiratory Cell and Molecular Biology, 25, 515–521.
Rengasamy, A., Barger, M. W., Kane, E., Ma, J. K., Castranova, V., & Ma, J. Y. (2003). Diesel exhaust particle-induced alterations of pulmonary phase I and phase II enzymes of rats. Journal of Toxicology and Environmental Health, 66, 153–167.
Bradley, J. M., Cryar, K. A., El Hajj, M. C., El Hajj, E. C., & Gardner, J. D. (2013). Exposure to diesel exhaust particulates induces cardiac dysfunction and remodeling. Journal of Applied Physiology (1985), 115, 1099–1106.
Landvik, N. E., Gorria, M., Arlt, V. M., Asare, N., Solhaug, A., Lagadic-Gossmann, D., & Holme, J. A. (2007). Effects of nitrated-polycyclic aromatic hydrocarbons and diesel exhaust particle extracts on cell signalling related to apoptosis: Possible implications for their mutagenic and carcinogenic effects. Toxicology, 231, 159–174.
Risom, L., Moller, P., & Loft, S. (2005). Oxidative stress-induced DNA damage by particulate air pollution. Mutation Research, 592, 119–137.
Monks, T. J., & Lau, S. S. (1992). Toxicology of quinone-thioethers. Critical Reviews in Toxicology, 22, 243–270.
Valavanidis, A., Vlachogianni, T., Fiotakis, K., & Loridas, S. (2013). Pulmonary oxidative stress, inflammation and cancer: Respirable particulate matter, fibrous dusts and ozone as major causes of lung carcinogenesis through reactive oxygen species mechanisms. International Journal of Environmental Research and Public Health, 10, 3886–3907.
Chuang, H. C., Cheng, Y. L., Lei, Y. C., Chang, H. H., & Cheng, T. J. (2013). Protective effects of pulmonary epithelial lining fluid on oxidative stress and DNA single-strand breaks caused by ultrafine carbon black, ferrous sulphate and organic extract of diesel exhaust particles. Toxicology and Applied Pharmacology, 266, 329–334.
Fredenburgh, L. E., Perrella, M. A., & Mitsialis, S. A. (2007). The role of heme oxygenase-1 in pulmonary disease. American Journal of Respiratory Cell and Molecular Biology, 36, 158–165.
Chen, X. L., Varner, S. E., Rao, A. S., Grey, J. Y., Thomas, S., Cook, C. K., et al. (2003). Laminar flow induction of antioxidant response element-mediated genes in endothelial cells. A novel anti-inflammatory mechanism. Journal of Biological Chemistry, 278, 703–711.
Hsieh, C. Y., Hsiao, H. Y., Wu, W. Y., Liu, C. A., Tsai, Y. C., Chao, Y. J., et al. (2009). Regulation of shear-induced nuclear translocation of the Nrf2 transcription factor in endothelial cells. Journal of Biomedical Science, 16, 12.
Chao, M. W., Po, I. P., Laumbach, R. J., Koslosky, J., Cooper, K., & Gordon, M. K. (2012). DEP induction of ROS in capillary-like endothelial tubes leads to VEGF-A expression. Toxicology, 297, 34–46.
Hengstler, J. G., & Bolt, H. M. (2008). Oxidative stress: From modification of cell-cycle related events, secondary messenger function, dysregulation of small GTPases, protein kinases and phosphatases to redox-sensitive cancer models. Archives of Toxicology, 82, 271–272.
Tseng, C. Y., Wang, J. S., Chang, Y. J., Chang, J. F., & Chao, M. W. (2015). Exposure to high-dose diesel exhaust particles induces intracellular oxidative stress and causes endothelial apoptosis in cultured in vitro capillary tube cells. Cardiovascular Toxicology, 15, 345–354.
Azad, M. B., Chen, Y., & Gibson, S. B. (2009). Regulation of autophagy by reactive oxygen species (ROS): Implications for cancer progression and treatment. Antioxidants & Redox Signaling, 11, 777–790.
Li, Y. J., Kawada, T., & Azuma, A. (2013). Nrf2 is a protective factor against oxidative stresses induced by diesel exhaust particle in allergic asthma. Oxidative Medicine and Cellular Longevity, 2013, 323607.
Brouard, S., Berberat, P. O., Tobiasch, E., Seldon, M. P., Bach, F. H., & Soares, M. P. (2002). Heme oxygenase-1-derived carbon monoxide requires the activation of transcription factor NF-kappa B to protect endothelial cells from tumor necrosis factor-alpha-mediated apoptosis. The Journal of Biological Chemistry, 277, 17950–17961.
Silva, G., Cunha, A., Gregoire, I. P., Seldon, M. P., & Soares, M. P. (2006). The antiapoptotic effect of heme oxygenase-1 in endothelial cells involves the degradation of p38 alpha MAPK isoform. The Journal of Immunology, 177, 1894–1903.
Wang, Z., Armando, I., Asico, L. D., Escano, C., Wang, X., Lu, Q., et al. (2007). The elevated blood pressure of human GRK4gamma A142V transgenic mice is not associated with increased ROS production. American Journal of Physiology, 292, H2083–H2092.
Wilson, S. J., & Keenan, A. K. (2003). Role of hemin in the modulation of H2O2-mediated endothelial cell injury. Vascular Pharmacology, 40, 109–118.
Dulak, J., Loboda, A., & Jozkowicz, A. (2008). Effect of heme oxygenase-1 on vascular function and disease. Current Opinion in Lipidology, 19, 505–512.
Nagy, J. A., Vasile, E., Feng, D., Sundberg, C., Brown, L. F., Manseau, E. J., et al. (2002). VEGF-A induces angiogenesis, arteriogenesis, lymphangiogenesis, and vascular malformations. Cold Spring Harbor Symposia on Quantitative Biology, 67, 227–237.
Weis, S. M., & Cheresh, D. A. (2005). Pathophysiological consequences of VEGF-induced vascular permeability. Nature, 437, 497–504.
Vandenbroucke, E., Mehta, D., Minshall, R., & Malik, A. B. (2008). Regulation of endothelial junctional permeability. Annals of the New York Academy of Sciences, 1123, 134–145.
Auger, F., Gendron, M. C., Chamot, C., Marano, F., & Dazy, A. C. (2006). Responses of well-differentiated nasal epithelial cells exposed to particles: Role of the epithelium in airway inflammation. Toxicology and Applied Pharmacology, 215, 285–294.
Jalava, P., Salonen, R. O., Halinen, A. I., Sillanpaa, M., Sandell, E., & Hirvonen, M. R. (2005). Effects of sample preparation on chemistry, cytotoxicity, and inflammatory responses induced by air particulate matter. Inhalation Toxicology, 17, 107–117.
Veranth, J. M., Cutler, N. S., Kaser, E. G., Reilly, C. A., & Yost, G. S. (2008). Effects of cell type and culture media on Interleukin-6 secretion in response to environmental particles. Toxicology in Vitro, 22, 498–509.
Rusznak, C., Mills, P. R., Devalia, J. L., Sapsford, R. J., Davies, R. J., & Lozewicz, S. (2000). Effect of cigarette smoke on the permeability and IL-1beta and sICAM-1 release from cultured human bronchial epithelial cells of never-smokers, smokers, and patients with chronic obstructive pulmonary disease. American Journal of Respiratory Cell and Molecular Biology, 23, 530–536.
Eleuteri, E., Magno, F., Gnemmi, I., Carbone, M., Colombo, M., La Rocca, G., et al. (2009). Role of oxidative and nitrosative stress biomarkers in chronic heart failure. Frontiers in Bioscience, 14, 2230–2237.
De Biase, L., Pignatelli, P., Lenti, L., Tocci, G., Piccioni, F., Riondino, S., et al. (2003). Enhanced TNF alpha and oxidative stress in patients with heart failure: Effect of TNF alpha on platelet O2-production. Thrombosis and Haemostasis, 90, 317–325.
Baulig, A., Garlatti, M., Bonvallot, V., Marchand, A., Barouki, R., Marano, F., & Baeza-Squiban, A. (2003). Involvement of reactive oxygen species in the metabolic pathways triggered by diesel exhaust particles in human airway epithelial cells. American Journal of Physiology, 285, L671–L679.
Tornqvist, H., Mills, N. L., Gonzalez, M., Miller, M. R., Robinson, S. D., Megson, I. L., et al. (2007). Persistent endothelial dysfunction in humans after diesel exhaust inhalation. American Journal of Respiratory and Critical Care Medicine, 176, 395–400.
Forchhammer, L., Loft, S., Roursgaard, M., Cao, Y., Riddervold, I. S., Sigsgaard, T., & Moller, P. (2012). Expression of adhesion molecules, monocyte interactions and oxidative stress in human endothelial cells exposed to wood smoke and diesel exhaust particulate matter. Toxicology Letters, 209, 121–128.
Yokota, S., Ohara, N., & Kobayashi, T. (2008). The effects of organic extract of diesel exhaust particles on ischemia/reperfusion-related arrhythmia and on pulmonary inflammation. Journal of Toxicological Sciences, 33, 1–10.
Nwariaku, F. E., Liu, Z., Zhu, X., Turnage, R. H., Sarosi, G. A., & Terada, L. S. (2002). Tyrosine phosphorylation of vascular endothelial cadherin and the regulation of microvascular permeability. Surgery, 132, 180–185.
Cromer, W. E., Zawieja, S. D., Tharakan, B., Childs, E. W., Newell, M. K., & Zawieja, D. C. (2014). The effects of inflammatory cytokines on lymphatic endothelial barrier function. Angiogenesis, 17, 395–406.
Maruo, N., Morita, I., Shirao, M., & Murota, S. (1992). IL-6 increases endothelial permeability in vitro. Endocrinology, 131, 710–714.
Chao, M. W., Kozlosky, J., Po, I. P., Strickland, P. O., Svoboda, K. K., Cooper, K., et al. (2011). Diesel exhaust particle exposure causes redistribution of endothelial tube VE-cadherin. Toxicology, 279, 73–84.
Higgins, K. J., Jung, H., Kittelson, D. B., Roberts, J. T., & Zachariah, M. R. (2003). Kinetics of diesel nanoparticle oxidation. Environmental Science and Technology, 37, 1949–1954.
Burch, W. M. (2002). Passage of inhaled particles into the blood circulation in humans. Circulation, 106, e141–E142. (author reply e141–142).
Corada, M., Liao, F., Lindgren, M., Lampugnani, M. G., Breviario, F., Frank, R., et al. (2001). Monoclonal antibodies directed to different regions of vascular endothelial cadherin extracellular domain affect adhesion and clustering of the protein and modulate endothelial permeability. Blood, 97, 1679–1684.
Harris, E. S., & Nelson, W. J. (2010). VE-cadherin: At the front, center, and sides of endothelial cell organization and function. Current Opinion in Cell Biology, 22, 651–658.
Gavard, J. (2013). Endothelial permeability and VE-cadherin: A wacky comradeship. Cell Adhesion & Migration, 7, 455–461.
Dudek, S. M., & Garcia, J. G. (2001). Cytoskeletal regulation of pulmonary vascular permeability. Journal of Applied Physiology, 91, 1487–1500.
Tseng, C. Y., Chang, J. F., Wang, J. S., Chang, Y. J., & Chao, M. W. (2015). Protective effects of N-acetyl cysteine against diesel exhaust particles-induced intracellular ROS generates pro-inflammatory cytokines to mediate the vascular permeability of capillary-like endothelial tubes. PLoS One, 10, e0131911.
Chow, J. C., Watson, J. G., Mauderly, J. L., Costa, D. L., Wyzga, R. E., Vedal, S., et al. (2006). Health effects of fine particulate air pollution: Lines that connect. Journal of the Air & Waste Management Association (1995), 56, 1368–1380.
Choy, J. C., Granville, D. J., Hunt, D. W., & McManus, B. M. (2001). Endothelial cell apoptosis: Biochemical characteristics and potential implications for atherosclerosis. Journal of Molecular and Cellular Cardiology, 33, 1673–1690.
Kerr, J. F., Wyllie, A. H., & Currie, A. R. (1972). Apoptosis: A basic biological phenomenon with wide-ranging implications in tissue kinetics. British Journal of Cancer, 26, 239–257.
Shen, B., He, P. J., & Shao, C. L. (2013). Norcantharidin induced DU145 cell apoptosis through ROS-mediated mitochondrial dysfunction and energy depletion. PLoS One, 8, e84610.
Rubtsova, S. N., Kondratov, R. V., Kopnin, P. B., Chumakov, P. M., Kopnin, B. P., & Vasiliev, J. M. (1998). Disruption of actin microfilaments by cytochalasin D leads to activation of p53. FEBS Letters, 430, 353–357.
White, S. R., Williams, P., Wojcik, K. R., Sun, S., Hiemstra, P. S., Rabe, K. F., & Dorscheid, D. R. (2001). Initiation of apoptosis by actin cytoskeletal derangement in human airway epithelial cells. American Journal of Respiratory Cell and Molecular Biology, 24, 282–294.
Carmeliet, P., Lampugnani, M. G., Moons, L., Breviario, F., Compernolle, V., Bono, F., et al. (1999). Targeted deficiency or cytosolic truncation of the VE-cadherin gene in mice impairs VEGF-mediated endothelial survival and angiogenesis. Cell, 98, 147–157.
Kerr, B. A., Ma, L., West, X. Z., Ding, L., Malinin, N. L., Weber, M. E., et al. (2013). Interference with akt signaling protects against myocardial infarction and death by limiting the consequences of oxidative stress. Science Signaling, 6, ra67.
Robertson, S., Thomson, A. L., Carter, R., Stott, H. R., Shaw, C. A., Hadoke, P. W., et al. (2014). Pulmonary diesel particulate increases susceptibility to myocardial ischemia/reperfusion injury via activation of sensory TRPV1 and beta1 adrenoreceptors. Particle and Fibre Toxicology, 11, 12.
Liuzzo, G. (2001). Atherosclerosis: an inflammatory disease. Rays, 26, 221–230.
Bochaton-Piallat, M. L., Gabbiani, F., Redard, M., Desmouliere, A., & Gabbiani, G. (1995). Apoptosis participates in cellularity regulation during rat aortic intimal thickening. The American Journal of Pathology, 146, 1059–1064.
Nussenzweig, S. C., Verma, S., & Finkel, T. (2015). The role of autophagy in vascular biology. Circulation Research, 116, 480–488.
Benbrook, D. M., & Long, A. (2012). Integration of autophagy, proteasomal degradation, unfolded protein response and apoptosis. Experimental Oncology, 34, 286–297.
Gustafsson, A. B., & Gottlieb, R. A. (2008). Recycle or die: The role of autophagy in cardioprotection. Journal of Molecular and Cellular Cardiology, 44, 654–661.
Yogalingam, G., & Pendergast, A. M. (2008). Abl kinases regulate autophagy by promoting the trafficking and function of lysosomal components. The Journal of Biological Chemistry, 283, 35941–35953.
Tsukahara, T., Matsuda, Y., Usui, Y., & Haniu, H. (2013). Highly purified, multi-wall carbon nanotubes induce light-chain 3B expression in human lung cells. Biochemical and Biophysical Research Communications, 440, 348–353.
Koehler, A., Marx, U., Broeg, K., Bahns, S., & Bressling, J. (2008). Effects of nanoparticles in Mytilus edulis gills and hepatopancreas—A new threat to marine life? Marine Environmental Research, 66, 12–14.
Halamoda Kenzaoui, B., Chapuis Bernasconi, C., Guney-Ayra, S., & Juillerat-Jeanneret, L. (2012). Induction of oxidative stress, lysosome activation and autophagy by nanoparticles in human brain-derived endothelial cells. The Biochemical Journal, 441, 813–821.
Kobayashi, S., Kojidani, T., Osakada, H., Yamamoto, A., Yoshimori, T., Hiraoka, Y., & Haraguchi, T. (2010). Artificial induction of autophagy around polystyrene beads in nonphagocytic cells. Autophagy, 6, 36–45.
Roy, R., Singh, S. K., Chauhan, L. K., Das, M., Tripathi, A., & Dwivedi, P. D. (2014). Zinc oxide nanoparticles induce apoptosis by enhancement of autophagy via PI3K/Akt/mTOR inhibition. Toxicology Letters, 227, 29–40.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tseng, CY., Wang, JS. & Chao, MW. Causation by Diesel Exhaust Particles of Endothelial Dysfunctions in Cytotoxicity, Pro-inflammation, Permeability, and Apoptosis Induced by ROS Generation. Cardiovasc Toxicol 17, 384–392 (2017). https://doi.org/10.1007/s12012-016-9364-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12012-016-9364-0