Abstract
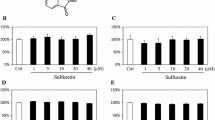
The transforming growth factors β1 (TGF-β1) and TGF-β2, as two distinct homodimers of TGF-β superfamily, involve in chondrocyte growth and differentiation. Emerging evidence has implied that strontium (Sr) plays an important role in the bone formation and resorption, and has strong effects on stimulating human cartilage matrix formation in vitro. However, the direct effects of Sr on TGF-β1 and TGF-β2 expressions in chondrocytes are not entirely clear. The purpose of this study was to evaluate the influence of different Sr concentrations on the expression of TGF-β1 and TGF-β2 in rat chondrocytes in vitro. Chondrocytes were isolated from Wistar rat articular by enzymatic digestion. Strontium chloride hexahydrate (SrCl2·6H2O) was used as a Sr source in this study. Sr was added to the culture solution at final concentrations of 0, 0.5, 1.0, 2.0, 5.0, 20.0, and 100 μg/mL. After 72 h of continuous culture, TGF-β1 and TGF-β2 mRNA abundance and protein expression levels in the chondrocytes were determined by real-time polymerase chain reaction (real-time PCR) and Western blot, respectively. The results showed that TGF-β1 and TGF-β2 expressions in chondrocytes increased dose-dependently with Sr concentration. The mRNA abundance of TGF-β1 and TGF-β2 were markedly higher than those observed for control (P < 0.01) when the Sr-treated concentration exceeded 1.0 and 5.0 μg/mL, respectively. The TGF-β1 and TGF-β2 protein expression levels were extremely significantly higher than those in the control group (P < 0.01) at above 5.0 μg/mL Sr-treatment. These results indicated that Sr could involve in the chondrocytes metabolism via regulating TGF-β1 and TGF-β2 signalling.


Similar content being viewed by others
References
Li TF, O’Keefe RJ, Chen D (2005) TGF-β signaling in chondrocytes. Front Biosci 10(1–3):681–688. https://doi.org/10.2741/1563
Kuhn AR, Das R, Pavanram P, Pufe T, Jahr H (2016) TGF-β superfamily members preserve the chondrocyte phenotype under physioxia in vitro. J Orthop Transl 7(C):109–110. https://doi.org/10.1016/j.jot.2016.06.197
Lafont J, Jacques C, Le Dreau G, Calhabeu F, Thibout H, Dubois C, Berenbaum F, Laurent M, Martinerie C (2005) New target genes for NOV/CCN3 in chondrocytes: TGF-β2 and type X collagen. J Bone Miner Res 20(12):2213–2223. https://doi.org/10.1359/JBMR.050818
Zhao Y, Guo D, Hou S, Zhong H, Yan J, Zhang C, Zhou Y (2013) Porous allograft bone scaffolds: doping with strontium. PLoS One 8(7):e69339. https://doi.org/10.1371/journal.pone.0069339
Cabrera WE, Schrooten I, De Broe ME, D'haese PC (1999) Strontium and bone. J Bone Miner Res 14(5):661–668. https://doi.org/10.1359/jbmr.1999.14.5.661
Schrooten I, Cabrera W, Goodman WG, Dauwe S, Lamberts LV, Marynissen R, Dorriné W, De Broe ME, D’Haese PC (1998) Strontium causes osteomalacia in chronic renal failure rats. Kidney Int 54(2):448–456. https://doi.org/10.1046/j.1523-1755.1998.00035.x
Yang F, Yang D, Tu J, Zheng Q, Cai L, Wang L (2011) Strontium enhances osteogenic differentiation of mesenchymal stem cells and in vivo bone formation by activating Wnt/catenin signaling. Stem Cells 29(6):981–991. https://doi.org/10.1002/stem.646
Ammann P (2006) Strontium ranelate: a physiological approach for an improved bone quality. Bone 38(2):15–18. https://doi.org/10.1016/j.bone.2005.09.023
Marie P (2006) Strontium ranelate: a physiological approach for optimizing bone formation and resorption. Bone 38(2):10–14. https://doi.org/10.1016/j.bone.2005.07.029
Marie P (2005) Strontium ranelate: a novel mode of action optimizing bone formation and resorption. Osteoporos Int 16(1):S7–S10. https://doi.org/10.1007/s00198-004-1753-8
Wang J, Zhu X, Liu L, Shi X, Yin L, Zhang Y, Li X, Wang Z, Liu G (2013) Effects of strontium on collagen content and expression of related genes in rat chondrocytes cultured in vitro. Biol Trace Elem Res 153(1–3):212–219. https://doi.org/10.1007/s12011-013-9640-9
Henrotin Y, Labasse A, Zheng S, Galais P, Tsouderos Y, Crielaard J-M, Reginster J-Y (2001) Strontium ranelate increases cartilage matrix formation. J Bone Miner Res 16(2):299–308. https://doi.org/10.1359/jbmr.2001.16.2.299
Karsenty G, Wagner EF (2002) Reaching a genetic and molecular understanding of skeletal development. Dev Cell 2(4):389–406. https://doi.org/10.1016/S1534-5807(02)00157-0
Horton WA (2003) Skeletal development: insights from targeting the mouse genome. Lancet 362(9383):560–569. https://doi.org/10.1016/S0140-6736(03)14119-0
Canalis E, Hott M, Deloffre P, Tsouderos Y, Marie P (1996) The divalent strontium salt S12911 enhances bone cell replication and bone formation in vitro. Bone 18(6):517–523. https://doi.org/10.1016/8756-3282(96)00080-4
Takahashi N, Sasaki T, Tsouderos Y, Suda T (2003) S 12911-2 inhibits osteoclastic bone resorption in vitro. J Bone Miner Res 18(6):1082–1087. https://doi.org/10.1359/jbmr.2003.18.6.1082
Morabito N, Catalano A, Gaudio A, Morini E, Bruno LM, Basile G, Tsiantouli E, Bellone F, Agostino RM, Piraino B (2016) Effects of strontium ranelate on bone mass and bone turnover in women with thalassemia major-related osteoporosis. J Bone Miner Metab 34(5):540–546. https://doi.org/10.1007/s00774-015-0689-8
Alexandersen P, Karsdal M, Qvist P, Reginster J-Y, Christiansen C (2007) Strontium ranelate reduces the urinary level of cartilage degradation biomarker CTX-II in postmenopausal women. Bone 40(1):218–222. https://doi.org/10.1016/j.bone.2006.07.028
Shi Y, Massagué J (2003) Mechanisms of TGF-β signaling from cell membrane to the nucleus. Cell 113(6):685–700. https://doi.org/10.1016/S0092-8674(03)00432-X
Ikushima H, Miyazono K (2012) TGF-β signal transduction spreading to a wider field: a broad variety of mechanisms for context-dependent effects of TGF-β. Cell Tissue Res 347(1):37–49. https://doi.org/10.1007/s00441-011-1179-5
Tang Y, Xiao J, Wang Y, Li M, Shi Z (2017) Effect of adenovirus-mediated TGF-β1 gene transfer on the function of rabbit articular chondrocytes. J Orthop Sci 22(1):149–155. https://doi.org/10.1016/j.jos.2016.05.009
Hickery MS, Bayliss MT, Dudhia J, Lewthwaite JC, Edwards JC, Pitsillides AA (2003) Age-related changes in the response of human articular cartilage to IL-1α and transforming growth factor β (TGF-β) chondrocytes exhibit a diminished sensitivity to TGF-β. J Biol Chem 278(52):53063–53071. https://doi.org/10.1074/jbc.M209632200
Worster AA, Nixon AJ, Brower-Toland BD, Williams J (2000) Effect of transforming growth factor β1 on chondrogenic differentiation of cultured equine mesenchymal stem cells. Am J Vet Res 61(9):1003–1010. https://doi.org/10.2460/ajvr.2000.61.1003
Das R, Timur U, Edip S, Haak E, Wruck C, Weinans H, Jahr H (2015) TGF-β2 is involved in the preservation of the chondrocyte phenotype under hypoxic conditions. Ann Anat 198:1–10. https://doi.org/10.1016/j.aanat.2014.11.003
Davies LC, Blain EJ, Gilbert SJ, Caterson B, Duance VC (2008) The potential of IGF-1 and TGF-β1 for promoting “adult” articular cartilage repair: an in vitro study. Tissue Eng Part A 14(7):1251–1261. https://doi.org/10.1089/ten.tea.2007.0211
Xu R, Li J, Wei B, Huo W, Wang L (2017) MicroRNA-483-5p modulates the expression of cartilage-related genes in human chondrocytes through down-regulating TGF-β1 expression. Tohoku J Exp Med 243(1):41–48. https://doi.org/10.1620/tjem.243.41
Hui W, Rowan AD, Cawston T (2001) Modulation of the expression of matrix metalloproteinase and tissue inhibitors of metalloproteinases by TGF-β1 and IGF-1 in primary human articular and bovine nasal chondrocytes stimulated with TNF-α. Cytokine 16(1):31–35. https://doi.org/10.1006/cyto.2001.0950
Wang W, Lou S, Ju X, Xia K, Xia J (2003) In vitro chondrogenesis of human bone marrow-derived mesenchymal progenitor cells in monolayer culture: activation by transfection with TGF-β2. Tissue Cell 35(1):69–77. https://doi.org/10.1016/S0040-8166(02)00106-4
Van Osch G, van der Veen SW, Verwoerd-Verhoef HL (2001) In vitro redifferentiation of culture-expanded rabbit and human auricular chondrocytes for cartilage reconstruction. Plast Reconstr Surg 107(2):433–440. https://doi.org/10.1097/00006534-200102000-00020
Leonard CM, Fuld HM, Frenz DA, Downie SA, Massague J, Newman SA (1991) Role of transforming growth factor β in chondrogenic pattern formation in the embryonic limb: stimulation of mesenchymal condensation and fibronectin gene expression by exogenenous TGF-β and evidence for endogenous TGF-β-like activity. Dev Biol 145(1):99–109. https://doi.org/10.1016/0012-1606(91)90216-P
Roark EF, Greer K (1994) Transforming growth factor β and bone morphogenetic protein-2 act by distinct mechanisms to promote chick limb cartilage differentiation in vitro. Dev Dynam 200(2):103–116. https://doi.org/10.1002/aja.1002000203
Sherwood JC, Bertrand J, Eldridge SE, Dell'Accio F (2014) Cellular and molecular mechanisms of cartilage damage and repair. Drug Discov Today 19(8):1172–1177. https://doi.org/10.1016/j.drudis.2014.05.014
Heldin CH, Miyazono K, tenDijke P (1997) TGF-beta signalling from cell membrane to nucleus through SMAD proteins. Nature 390(6659):465–471. https://doi.org/10.1038/37284
Li M, Wang Y, Liao N, Li J, Dong Q (2017) Changes of TGF-β1 expression during orthodontic tooth movement in rats with osteoporosis. Shanghai J Stomatol 26(1):17–20
Huang X, Lv H, Jin S, Guo R, Wu W (2013) Strontium ranelate promotes osteogenic differentiation of rat bone mesenchymal stem cells through TGF-β1/Smad signaling pathway. Chinese J Pathophysiol 29(2):302–307
Funding
The project was supported by the National Natural Science Foundation of China (Grant No. 31502129), China Postdoctoral Science Foundation funded project (No. 2014 M560811), and Programs for Science and Technology Shaanxi (No. 2016NY-100).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors alone are responsible for the content and writing of the article. The study was approved by the Institutional Animal Research Committee Guidelines of Northwest A&F University in China.
Conflict of Interest
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Kong, Y., Guo, Y., Zhang, J. et al. Strontium Promotes Transforming Growth Factors β1 and β2 Expression in Rat Chondrocytes Cultured In Vitro. Biol Trace Elem Res 184, 450–455 (2018). https://doi.org/10.1007/s12011-017-1208-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-017-1208-7