Abstract
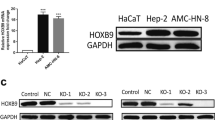
Lung squamous cell carcinoma (LUSC) is one subtype of non-small-cell lung cancer, whose pathogenesis has not been fully understood. Exploring molecular mechanisms of LUSC helps a lot with the development of LUSC novel therapy. Hence, our study aims to investigate novel molecular mechanisms. Differentially expressed miRNAs and mRNAs were acquired from The Cancer Genome Atlas database. A series of assays were applied to test cell functions, including qRT-PCR to analyze RND1 and miR-4652-5p expression, dual-luciferase reporter gene assay to verify the targeting relationship between these two genes, cell counting kit-8 and colony formation assays to evaluate the ability of LUSC cells to proliferate, transwell to examine the migratory and invasive abilities, and western blot to test expression of RND1 and cell adhesion-related proteins. RND1 was lowly expressed while miR-4652-5p was highly expressed in LUSC cells. The correlation between these two genes was significantly negative and miR-4652-5p could downregulate RND1 expression. Additionally, cellular function assays validated that RND1 suppressed LUSC cells to proliferate, migrate, and invade. Besides, this gene might also affect cell adhesion. Furthermore, rescue assay suggested that miR-4652-5p downregulated RND1 expression to promote the progression of LUSC cells. Together, miR-4652-5p targeted RND1 to modulate cell adhesion and the progression of LUSC cells.




Similar content being viewed by others
Data Availability
The data and materials in the current study are available from the corresponding author on reasonable request.
References
Conti, L., & Gatt, S. (2018). Squamous-cell carcinoma of the lung. New England Journal of Medicine, 379, e17. https://doi.org/10.1056/NEJMicm1802514
Gao, M., Kong, W., Huang, Z. & Xie, Z. (2020) Identification of key genes related to lung squamous cell carcinoma using bioinformatics analysis. International Journal of Molecular Sciences 21, https://doi.org/10.3390/ijms21082994.
Xu, F., Zhang, H., Chen, J., Lin, L., & Chen, Y. (2020). Immune signature of T follicular helper cells predicts clinical prognostic and therapeutic impact in lung squamous cell carcinoma. International Immunopharmacology, 81, 105932. https://doi.org/10.1016/j.intimp.2019.105932
Mouly, L. et al. (2019) The RND1 small GTPase: Main functions and emerging role in oncogenesis. International Journal of Molecular Sciences 20, https://doi.org/10.3390/ijms20153612.
Okada, T., et al. (2015). The Rho GTPase Rnd1 suppresses mammary tumorigenesis and EMT by restraining Ras-MAPK signalling. Nature Cell Biology, 17, 81–94. https://doi.org/10.1038/ncb3082
Qin, C. D., et al. (2018). The Rho GTPase Rnd1 inhibits epithelial-mesenchymal transition in hepatocellular carcinoma and is a favorable anti-metastasis target. Cell Death & Disease, 9, 486. https://doi.org/10.1038/s41419-018-0517-x
Chen, X., Koh, E., Yoder, M., & Gumbiner, B. M. (2009). A protocadherin-cadherin-FLRT3 complex controls cell adhesion and morphogenesis. PLoS ONE, 4, e8411. https://doi.org/10.1371/journal.pone.0008411
Ogata, S., et al. (2007). TGF-beta signaling-mediated morphogenesis: Modulation of cell adhesion via cadherin endocytosis. Genes & Development, 21, 1817–1831. https://doi.org/10.1101/gad.1541807
Li, B., et al. (2020). WNT1, a target of miR-34a, promotes cervical squamous cell carcinoma proliferation and invasion by induction of an E-P cadherin switch via the WNT/beta-catenin pathway. Cellular Oncology (Dordrecht), 43, 489–503. https://doi.org/10.1007/s13402-020-00506-8
Mitra, R., Adams, C. M., Jiang, W., Greenawalt, E., & Eischen, C. M. (2020). Pan-cancer analysis reveals cooperativity of both strands of microRNA that regulate tumorigenesis and patient survival. Nature Communications, 11, 968. https://doi.org/10.1038/s41467-020-14713-2
Jiang, C., Hu, X., Alattar, M., & Zhao, H. (2014). miRNA expression profiles associated with diagnosis and prognosis in lung cancer. Expert Review of Anticancer Therapy, 14, 453–461. https://doi.org/10.1586/14737140.2013.870037
Lu, J., et al. (2005). MicroRNA expression profiles classify human cancers. Nature, 435, 834–838. https://doi.org/10.1038/nature03702
Chen, C., Tang, J., Xu, S., Zhang, W., & Jiang, H. (2020). miR-30a-5p inhibits proliferation and migration of lung squamous cell carcinoma cells by targeting FOXD1. BioMed Research International, 2020, 2547902. https://doi.org/10.1155/2020/2547902
Qiao, G., Wang, H. B., Duan, X. N., & Yan, X. F. (2021). The effect and mechanism of miR-607/CANT1 axis in lung squamous carcinoma. Anti-Cancer Drugs, 32, 693–702. https://doi.org/10.1097/CAD.0000000000001045
Wu, Y., et al. (2020). miR-1301-3p promotes the proliferation and migration of lung cancer cells via direct repression of polymerase I and transcript release factor. Oncology Letters, 20, 286. https://doi.org/10.3892/ol.2020.12149
Robinson, M. D., McCarthy, D. J., & Smyth, G. K. (2010). edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics, 26, 139–140. https://doi.org/10.1093/bioinformatics/btp616
Boyrie, S., et al. (2018). RND1 regulates migration of human glioblastoma stem-like cells according to their anatomical localization and defines a prognostic signature in glioblastoma. Oncotarget, 9, 33788–33803. https://doi.org/10.18632/oncotarget.26082
Cheng, Z., et al. (2019). circTP63 functions as a ceRNA to promote lung squamous cell carcinoma progression by upregulating FOXM1. Nature Communications, 10, 3200. https://doi.org/10.1038/s41467-019-11162-4
Fan, X., et al. (2020). Long non-coding RNA LINC01116 regulated miR-744-5p/SCN1B axis to exacerbate lung squamous cell carcinoma. Cancer Biomarkers, 28, 473–482. https://doi.org/10.3233/CBM-190945
Wang, Z., et al. (2019). Identification of seven-gene signature for prediction of lung squamous cell carcinoma. Oncotargets and Therapy, 12, 5979–5988. https://doi.org/10.2147/OTT.S198998
Shen, L., et al. (1842). Overexpression of Oct4 suppresses the metastatic potential of breast cancer cells via Rnd1 downregulation. Biochimica et Biophysica Acta, 2087–2095, 2014. https://doi.org/10.1016/j.bbadis.2014.07.015
Yang, C., Dou, R., Yin, T., & Ding, J. (2020). MiRNA-106b-5p in human cancers: Diverse functions and promising biomarker. Biomedicine & Pharmacotherapy, 127, 110211. https://doi.org/10.1016/j.biopha.2020.110211
Wu, C., et al. (2020). Two miRNA prognostic signatures of head and neck squamous cell carcinoma: A bioinformatic analysis based on the TCGA dataset. Cancer Medicine, 9, 2631–2642. https://doi.org/10.1002/cam4.2915
Li, Y., et al. (2020). SPEN induces miR-4652-3p to target HIPK2 in nasopharyngeal carcinoma. Cell Death & Disease, 11, 509. https://doi.org/10.1038/s41419-020-2699-2
Author information
Authors and Affiliations
Contributions
Y. Z. and S. Y. contributed to the study design. J. Y. and H. C. conducted the literature search. W. Z. and G. X. acquired the data. H. Z. wrote the article. Y. Z. performed the data analysis and drafted. S. Y. revised the article. All the authors gave the final approval of the version to be submitted.
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
Not applicable.
Competing Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhou, Y., Yan, J., Chen, H. et al. MiR-4652-5p Targets RND1 to Regulate Cell Adhesion and Promote Lung Squamous Cell Carcinoma Progression. Appl Biochem Biotechnol 194, 3031–3043 (2022). https://doi.org/10.1007/s12010-022-03897-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-022-03897-6