Abstract
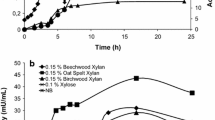
Xylanases are enzymes that act in the depolymerization of xylan and that can be used in the food industry, the paper industry, and for bioenergy, among other uses. In this context, particular emphasis is devoted to xylooligosaccharides (XOS) that act as prebiotics, which, under the action of probiotic microorganisms, are capable of positively modifying the intestinal microbiota. In this sense, searching for microbial xylanases stands out as a sustainable strategy for the production of prebiotics. To date, there have been no reports in the literature regarding the purification of native xylanase from Myceliophthora heterothallica F.2.1.4. In this study, a xylanase from this fungus was purified and characterized. The xylanase, with 27 kDa, showed maximum activity at pH 4.5 and 65–70 °C. It maintained more than 80% of its residual activity when exposed to (i) temperatures between 30 and 60 °C for 1 h and (ii) pH 5–10 for 24 h at 4 and 25 °C. These high tolerances to different pH and different temperatures are important properties that add value to this enzyme. The hydrolysates of this enzyme on beechwood xylan, analyzed by HPAE-PAD, were mostly xylobiose (X2) and xylotriose (X3). Hydrolysates were also quantified, being retrieved from 234.2 mg xylooligosaccharides/g of hydrolyzed xylan for 12 h. According to the products obtained from the xylan hydrolysis and its tolerance properties of the enzyme, it has demonstrated potential for application production of xylooligosaccharides for use as prebiotics.








Similar content being viewed by others
References
CAZy - Carbohydrate Active Enzymes database. Available from: www.cazy.org. Acessed June 26, 2018.
Polizeli, M. L. T. M., Rizzatti, A. C. S., Monti, R., Terenzi, H. F., Jorge, J. A., & Amorim, D. S. (2005). Xylanases from fungi: properties and industrial applications. Applied Microbiology and Biotechnology, 67(5), 577–591.
Verma, D., Anand, A., & Satyanarayana, T. (2013). Thermostable and alkalistable endoxylanase of the extremely thermophilic bacterium Geobacillus thermodenitrificans TSAA1: cloning, expression, characteristics and its applicability in generating xylooligosaccharides and fermentable sugars. Applied Biochemistry and Biotechnology, 170(1), 119–130.
Liu, X., Liu, Y., Jiang, Z., Liu, H., Yang, S., & Yan, Q. (2018). Biochemical characterization of a novel xylanase from Paenibacillus barengoltzii and its application in xylooligosaccharides production from corncobs. Food Chemistry, 264, 310–318.
Gibson, G. R., Hutkins, R., Sanders, M. E., Prescott, S. L., Reimer, R. A., Salminen, S. J., Scott, K., Stanton, C., Swanson, K. S., Cani, P. D., Verbeke, K., & Reid, G. (2017). The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nature Reviews. Gastroenterology & Hepatology, 14, 491–502.
Vrese, M. & Schrezenmeir, J. (2008). Probiotics, prebiotics, and synbiotics. Advances in Biochemical Engineering/Biotechnology, 111, 1–66.6.
Akpinar, O., Erdogan, K., & Bostanci, S. (2009). Enzymatic production of xylooligosaccharide from selected agricultural wastes. Food and Bioproducts Processing, 87(2), 145–151.
Vázquez, M. J., Alonso, J. L., Domínguez, H., & Parajó, J. C. (2002). Enzymatic processing of crude xylooligomer solutions obtained by autohydrolysis of eucalyptus wood. Food Biotechonology, 16, 91–105.
Brienzo, M., Carvalho, W., & Milagres, A. M. F. (2010). Xylooligosaccharides production from alkali-pretreated sugarcane bagasse using xylanases from Thermoascus aurantiacus. Applied Biochemistry and Biotechnology, 162(4), 1195–1205.
Bajpai, P (2014). Xylanolytic enzymes. Chapter 6 - purification of xylanases, Academic Press, 53–61.
Bhalla, A., Bansal, N., Kumar, S., Bischoff, K. M., & Sani, R. K. (2013). Improved lignocellulose conversion to biofuels with thermophilic bacteria and thermostable enzymes. Bioresource Technology, 128, 751–759.
Chapla, D., Pandit, P., & Shah, A. (2012). Production of xylooligosaccharides from corncob xylan by fungal xylanase and their utilization by prebiotics. Bioresource Technology, 115, 215–221.
Moretti, M. M. S., Bocchini-Martins, D. A., Silva, R., Rodrigues, A., Sette, L. D., & Gomes, E. (2012). Selection of thermophilic and thermotolerant fungi for the production of cellulases and xylanases under solid-state fermentation. Brazilian Journal of Microbiology, 43(3), 1062–1071.
Silva, V. C. T.; Coto, A. L. S.; Souza, R. C.; Neves, M. B. S.; Gomes, E. & Bonilla-Rodriguez, G. O (2016). Effect of pH, temperature, and chemicals on the endoglucanases and 훽-glucosidases from the thermophilic fungus Myceliophthora heterothallica F.2.1.4. Obtained by solid-state and submerged cultivation. Biochemistry Research International, 2016, 1–9.
Diaz, A. B., Moretti, M. M. S., Bezerra-Bussoli, C., Nunes, C. C. C., Blandino, A., Silva, R., & Gomes, E. (2015). Evaluation of microwave-assisted pretreatment of lignocellulosic biomass immersed in alkaline glycerol for fermentable sugars production. Bioresource Technology, 185, 316–323.
Zanphorlin, L. M., Facchini, F. D. A., Vasconcelos, F., Bonugli-Santos, R. C., Rodrigues, A., Sette, L. D., Gomes, E., & Bonilla-Rodriguez, G. O. (2010). Production, partial characterization, and immobilization in alginate beads of an alkaline protease from a new thermophilic fungus Myceliophthora sp. The Journal of Microbiology, 48(3), 331–336.
Bailey, M. J., Biely, P., & Poutanen, K. (1992). Interlaboratory testing of methods for assay of xylanase activity. Journal of Biotechnology, 23, 257–270.
Miller, G. L. (1959). Use of dinitrosalicylic acid reagent for determination of reducing sugar. Analytical Chemistry, 31(3), 426–429.
See, Y. S., & Jackowski, G. (1989). Estimating molecular weights of polypeptides by SDS gel electrophoresis. In T. E. Creigton (Ed.), Protein structure a practical approach (pp. 1–19). New York: Oxford University.
Silva, R. R., Caetano, R. C., Okamoto, D. N., Oliveira, L. C. G., Bertolin, T. C., Juliano, M. A., Juliano, L., Oliveira, A. H. C., Rosa, J. C., & Cabral, H. (2014). The identification and biochemical properties of the catalytic specificity of a serine peptidase secreted by Aspergillus fumigatus Fresenius. Protein and Peptide Letters, 21(7), 663–671.
Silva, R. R., Oliveira, L. C. G., Juliano, M. A., Juliano, L., Oliveira, A. H. C., Rosa, J. C., & Cabral, H. (2017a). Biochemical and milk-clotting properties and mapping of catalytic subsites of an extracellular aspartic peptidase from basidiomycete fungus Phanerochaete chrysosporium. Food Chemistry, 225, 45–54.
Silva, R. R., Oliveira, L. C. G., Juliano, M. A., Juliano, L., Rosa, J. C., & Cabral, H. (2017b). Activity of a peptidase secreted by Phanerochaete chrysosporium depends on lysine to subsite S’1. International Journal of Biological Macromolecules, 94, 474–483.
Noble, J. E. & Bailey, M. J. A. (2009). Quantification of protein. Methods in enzymology, pp. 81. In: Lorsch, J. (Ed.), Methods in enzymology. Academic Press, 463, 73–95.
Noble, J. E. (2014): Chapter two-quantification of protein concentration using UV absorbance and coomassie dyes, pp. 21. In: Lorsch, J. (Ed.), Methods in enzymology, Academic Press, 536.
McPhillips, K., Waters, D. M., Parlet, C., Walsh, D. J., Arendt, E. K., & Murray, P. G. (2014). Purification and characterisation of a β-1,4-xylanase from Remersonia thermophila CBS 540.69 and its application in bread making. Applied Biochemistry and Biotechnology, 172(4), 1747–1762.
Beaugrand, J., Chambat, G., Wong, V. W. K., Goubet, F., Rémond, C., Paës, G., Benamrouche, S., Debeire, P., O’Donohue, M., & Chabbert, B. (2004). Impact and efficiency of GH10 and GH11 thermostable endoxylanases on wheat bran and alkali-extractable arabinoxylans. Carbohydrate Research, 339(15), 2529–2540.
Sharma, M., Chadha, B. S., & Saini, H. S. (2010). Purification and characterization of two thermostable xylanases from Malbranchea flava active under alkaline conditions. Bioresource Technology, 101(22), 8834–8842.
Fawzi, E. M. (2011). Highly thermostable xylanase purified from Rhizomucor miehei NRL 3169. Acta Biologica Hungarica, 62(1), 85–94.
Basit, A., Liu, J., Miao, T., Zheng, F., Rahim, K., Lou, H., & Jiang, W. (2018). Characterization of two endo-β-1,4-xylanases from Myceliophthora thermophila and their saccharification efficiencies, synergistic with commercial cellulase. Frontiers in Microbiology, 9(233), 1–11.
Souza, A. R., Araújo, G. C., Zanphorlin, L. M., Ruller, R., Franco, F. C., Torres, F. A. G., Mertens, J. A., Bowman, M. J., Gomes, E., & Silva, R. (2016). Engineering increased thermostability in the GH-10 endo-1,4-β-xylanase from Thermoascus aurantiacus CBMAI 756. International Journal of Biological Macromolecules, 93(Pt A), 20–26.
Mosier, N., Wyman, C., Dale, B., Elander, R., Lee, Y. Y., Holtzapple, M., & Ladisch, M. (2005). Features of promising technologies for pretreatment of lignocellulosic biomass. Bioresource Technology, 96(6), 673–686.
Turunen, O., Etuaho, K., Fenel, F., Vehmaanpera, J., Wu, X., Rouvinen, J., & Leisola, M. (2001). A combination of weakly stabilizing mutations with a disulfide bridge in the α-helix region of Trichoderma reesei endo-1,4-β-xylanase II increases the thermal stability through synergism. Journal of Biotechnology, 88(1), 37–46.
Singh, B. (2014). Myceliophthora thermophila syn. Sporotrichum thermophile: a thermophilic mould of biotechnological potential. Critical Reviews in Biotechnology, 1–11.
Kannan, A.; Hettiarachchy, N. S. & Marshall, M. R. (2012). Food proteins - peptides. In: Hettiarachchy, N. S. et al. Food proteins and peptides chemistry, functionality, interactions, and commercialization, CRC Press, 1–24.
Al-Darkazali, H., Meevootisom, V., Isarangkul, D., & Wiyakrutta, S. (2017). Gene expression and molecular characterization of a xylanase from chicken cecum metagenome. International Journal of Microbiology, 2017, 1–12.
Zhao, H. (2005). Effect of ions and other compatible solutes on enzyme activity, and its implication for biocatalysis using ionic liquids. Review. Journal of Molecular Catalysis B Enzymatic, 37, 16–25, 1-6.
Mizrahi, L., & Achituv, Y. (1989). Effect of heavy metals ions on enzyme activity in the mediterranean mussel, Donax trunculus. Bulletin of Environmental Contamination and Toxicology, 42(6), 854–859.
Ding, C., Li, M., & Hu, Y. (2018). High-activity production of xylanase by Pichia stipitis: purification, characterization, kinetic evaluation and xylooligosaccharides production. Biological Macromolecules, 117, 72–77.
Michelin, M., Silva, T. M., Jorge, J. A., & Polizeli, M. L. T. M. (2014). Purification and biochemical properties of multiple xylanases from Aspergillus ochraceus tolerant to Hg2+ ion and a wide range of pH. Applied Biochemistry and Biotechnology, 174(1), 206–220.
Harris, A. D., & Ramalingam, C. (2010). Xylanases and its application in food industry: a review. Journal of Experimental Sciences, 1, 10–11.
Juturu, V., & Wu, J. C. (2012). Microbial xylanases: engineering, production and industrial applications. Biotechnology Advances, 30(6), 1219–1227.
Yang, H., Wang, K., Song, X., & Xu, F. (2011). Production of xylooligosaccharides by xylanase from Pichia stipitis based on xylan preparation from triploid Populas tomentosa. Bioresource Technology, 102(14), 7171–7176.
Otieno, D. O., & Ahring, B. K. (2012). The potential for oligosaccharide production from the hemicellulose fraction of biomasses through pretreatment processes: xylooligosaccharides (XOS), arabinooligosaccharides (AOS), and mannooligosaccharides (MOS). Carbohydrate Research, 360, 84–92.
Bragatto, J., Segato, F., & Squina, F. M. (2013). Production of xylooligosaccharides (XOS) from delignified sugarcane bagasse by peroxide-HAc process using recombinant xylanase from Bacillus subtilis. Industrial Crops and Products, 51, 123–129.
Samanta, A. K., Jayapal, N., Jayaram, C., Roy, S., Kolte, A. P., Senani, S., & Sridhar, M. (2015). Xylooligosaccharides as prebiotics from agricultural by-products: production and applications. Bioactive Carbohydrates and Dietary Fibre, 5(1), 62–71.
Linares-Pasten, J. A., Aronsson, A., & Karlsson, E. N. (2018). Structural considerations on the use of endo-xylanases for the production of prebiotic xylooligosaccharides from biomass. Current Protein and Peptide Science, 19(1), 48–67.
Hoffmam, Z. B., Zanphorlin, L. M., Cota, J., Diogo, J. A., Almeida, G. B., Damásio, A. R. L., Squina, F., Murakami, M. T., & Ruller, R. (2016). Xylan-specific carbohydrate-binding module belonging to family 6 enhances the catalytic performance of a GH11 endo-xylanase. New Biotechnology, 33(4), 467–472.
Santos, C. R., Hoffmam, Z. B., Martins, V. P. M., Zanphorlin, L. M., Assis, L. H. P., Honorato, R. V., Oliveira, P. S. L., Ruller, R., & Murakami, M. T. (2014). Molecular mechanisms associated with xylan degradation by xanthomonas plant pathogens. The Journal of Biological Chemistry, 289(46), 32186–32200.
Gullón, P., González-Muñoz, M. J., & Parajó, J. C. (2011). Manufacture and prebiotic potential of oligosaccharides derived from industrial solid wastes. Bioresource Technology, 102(10), 6112–6119.
Silva, R. R. (2017). Bacterial and fungal proteolytic enzymes, production, catalysis and potential applications. Applied Biochemistry and Biotechnology, 183(1), 1–19.48.
Silva, R. R., Pedezzi, R., & Souto, T. B. (2017). Exploring the bioprospecting and biotechnological potential of white-rot and anaerobic Neocallimastigomycota fungi: peptidases, esterases, and lignocellulolytic enzymes. Applied Microbiology and Biotechnology, 101(8), 3089–3101.
Acknowledgements
This work was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP), Process 2017/16482-5, and Conselho de Desenvolvimento Científico e Tecnológico (CNPq), Process 426578/2016-3. M. B, E.G, and R.S are research fellows of CNPq. The authors would like to thank Dr. Célia Maria Landi Franco and Dr. Márcia Maria de Souza Moretti for their help with the HPLC analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
de Oliveira Simões, L.C., da Silva, R.R., de Oliveira Nascimento, C.E. et al. Purification and Physicochemical Characterization of a Novel Thermostable Xylanase Secreted by the Fungus Myceliophthora heterothallica F.2.1.4. Appl Biochem Biotechnol 188, 991–1008 (2019). https://doi.org/10.1007/s12010-019-02973-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-019-02973-8