Abstract
Purpose of Review
To date, prostate cancer has been poorly responsive to immunotherapy. In the current review, we summarize and discuss the current literature on the use of vaccine therapy and checkpoint inhibitor immunotherapy in metastatic castration-resistant prostate cancer (mCRPC).
Recent Findings
Sipuleucel-T currently remains the only FDA-approved immunotherapeutic agent for prostate cancer. Single-agent phase 3 vaccine trials with GVAX and PROSTVAC have failed to demonstrate survival benefit to date. Clinical trials using combination approaches, including combination PROSTVAC along with a neoantigen vaccine and checkpoint inhibitor immunotherapy, are ongoing. Checkpoint inhibitor monotherapy clinical trials have demonstrated limited efficacy in advanced prostate cancer, and combination approaches and molecular patient selection are currently under investigation.
Summary
The optimal use of vaccine therapy and checkpoint inhibitor immunotherapy in metastatic castration-resistant prostate cancer remains to be determined. Ongoing clinical trials will continue to inform future clinical practice.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Prostate cancer remains the second most common cause of death among men in the USA, with an additional estimated 15,891 cases of metastatic prostate cancer by 2025 [1, 2]. Despite six FDA-approved therapies for metastatic castration-resistant prostate cancer (mCRPC), the clinical efficacy and utilization of immunotherapy agents are still in its relative infancy in this disease.
Harnessing the immune system to treat cancer has become a cornerstone of modern oncology therapeutics, with particular rapidity within the last two decades. In 2000, James Allison, Tasuku Honjo, and colleagues demonstrated an immune response in the prostate tumors of transgenic mice treated with a combination anti-cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) therapy and a granulocyte-macrophage colony-stimulating factor (GM-CSF) expressing vaccine [3], and had also seen similarly impressive results in a melanoma model [4]. Based on this, ipilimumab, an anti-cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) monoclonal antibody, eventually became the first checkpoint inhibitor immunotherapy drug to gain FDA approval in 2011 after a survival benefit was demonstrated in patients with metastatic melanoma [5]. Since that time, anti-PD-1 checkpoint inhibitors (pembrolizumab, nivolumab, cemiplimab) and anti-PD-L1 checkpoint inhibitors (atezolizumab, avelumab, durvalumab) have been approved across multiple malignancies [6,7,8,9,10]. Despite these important advances, patients with prostate cancer have not yet benefitted to as great an extent as those with more “immunologically responsive” cancers such as melanoma and urothelial carcinoma [11•, 12•]. Prostate cancers have historically been largely deemed immunologically “cold” tumors that generally have a lower tumor mutation burden (TMB) than other tumor types [13, 14]. The complexities of the prostate cancer immune milieu and tumor microenvironment (TME) are reviewed in other articles in this Special Edition, and our understanding of this biology will be critical for advancing the field of immunotherapy in prostate cancer. Here, we will focus on past vaccine-based and checkpoint inhibitor treatment modalities in prostate cancer, as well as emerging treatment approaches across both modalities.
Prior Approaches: Vaccines, Checkpoint Inhibitor TherapyVaccines
-
1.
Sipuleucel-T
Sipuleucel-T is a therapeutic cancer vaccine consisting of autologous antigen-presenting cells (APCs), stimulated with PA2024, a recombinant fusion protein of prostatic acid phosphatase (PAP) and granulocyte-macrophage colony-stimulating factor (GM-CSF). Several randomized, placebo-controlled trials had suggested a survival benefit, but no clear effect on progression free survival (PFS) [15, 16]. In 2010, Kantoff and colleagues conducted the phase 3 IMPACT trial of 512 men with metastatic castration-resistant prostate cancer, randomized in a 2:1 fashion to receive sipuleucel-T or placebo [17••]. The study met its primary endpoint of overall survival (OS), with a median survival of 25.8 months in the sipuleucel-T arm compared with 21.7 months in the control arm, a 4.1-month improvement. However, no difference in time to disease progression (PFS) or PSA response was observed, with only 2.6% of patients having a PSA decline of 50% or greater. However, based on the observed survival benefit, sipuleucel-T was FDA approved in 2010. Importantly, this was the first cellular therapeutic vaccine for any cancer type that was approved by the FDA, marking an important milestone in the field of cancer immunotherapy. Retrospective analysis of the IMPACT trial stratified patients by PSA levels, and found a 13-month improvement in OS in the lowest quartile compared with placebo, while the difference was only 2.8 months in the highest quartile, suggesting patients benefit the most at time of lowest tumor burden [18]. More recently, Holl et al. conducted a retrospective analysis of 336 patients with metastatic castration-resistant prostate cancer treated with sipuleucel-T. Interestingly, a subset of 44 patients showed PSA stabilization and long-term disease control, with 79% surviving 36 months with a median time to subsequent therapy of 17.8 months. There was a trend toward a higher percentage of African American (AA) patients falling into this category, compared with Caucasian counterparts [19]. Additional analyses of a prospective registry trial, PROCEED, found that AA patients had significantly longer median OS (39.5 months) compared with matched Caucasians (28.1 months; p < 0.001). AA race also emerged as an independent predictor of longer OS in multivariate analyses [20]. Taken together, these suggest that patient selection and host factors may be keys for optimizing responses to sipuleucel-T.
A recent randomized phase II trial of sipuleucel-T with or without sensitizing radiation therapy, administered to a single metastatic site up to 30 Gy, in patients with asymptomatic or minimally symptomatic mCRPC, did not enhance humoral and cellular responses to the vaccine therapy [21]. Several clinical trials have also evaluated the use of sipuleucel-T earlier in the course of prostate cancer. In a multicenter phase II trial of neoadjuvant sipuleucel-T administered to men with localized prostate cancer prior to planned radical prostatectomy (RP), 37 of 42 patients received three sipuleucel-T treatments. All 37 of the patients who received RP were found to have peripheral immune responses and immune infiltrates. CD3+, CD4+FOXP3-, and CD8+ T cell infiltrates were evident in the RP tissues of sipuleucel-T-treated patients and were concentrated primarily at the tumor interface. Nearly half of CD3+ T lymphocytes at the tumor interface of treated patients expressed PD-1 [22].
The ProVent trial (NCT03686683) is an ongoing randomized phase III, open-label clinical trial of sipuleucel-T administered to patients on active surveillance who have newly diagnosed prostate cancer, ISUP grade groups 1–3, and an estimated life expectancy of ≥ 10 years. Patients are randomized in a 2:1 fashion to receive sipuleucel-T or to standard of care active surveillance. The primary objective is to assess the efficacy of sipuleucel-T in reducing histopathologic reclassification to a higher Gleason grade in prostate cancer subjects on active surveillance 3 years after randomization. Such efforts are of value in this patient population given the progressive risk of distant metastases and prostate cancer–related mortality in patients with higher grade prostate cancer. Nonetheless, it is noteworthy that despite the documented survival benefit associated with sipuleucel-T, a recent large cohort study of its use in more than 7000 patients with metastatic castration-resistant prostate cancer found that only 1 of 10 patients receive this therapy, highlighting the relatively limited use of this therapy—the only FDA-approved immunotherapy for prostate cancer—in a real-world population [23].
-
2.
GVAX
GVAX is a GM-CSF-secreting tumor cell vaccine generated using LNCaP and PC-3 cell lines. A phase 1/2 study in patients with hormone-naïve prostate cancer and PSA relapse demonstrated a statistically significant decrease in PSA velocity in 16 of 21 patients (76%) at 20 weeks after first therapy [24]. However, two subsequent phase 3 clinical trials were terminated early based on the results of a previously unplanned futility analysis. VITAL-2 (NCT00133224) was a phase 3, randomized open-label study of docetaxel with or without GVAX in patients with taxane-naïve mCRPC, and VITAL-1 (NCT00089856) was a phase 3 clinical trial which randomized chemotherapy-naïve patients with mCRPC to receive either GVAX or docetaxel and prednisone. In VITAL-2, the combination GVAX and docetaxel arm was associated with an increased mortality rate compared with the chemotherapy monotherapy arm. This vaccine therapy has subsequently not been developed further and represents an important cautionary lesson in the importance of large, randomized phase 3 clinical trials to demonstrate therapeutic efficacy of novel drugs, particularly in the challenging immunotherapy landscape in prostate cancer.
-
3.
PROSTVAC
PROSTVAC-VF is an immunotherapeutic vaccine that incorporates the genes for prostate-specific antigen (PSA) and multiple T cell co-stimulatory molecules (TRICOM) in viral vectors to generate a T cell response in prostate cancer patients. It utilizes both recombinant vaccinia virus (“V”) and recombinant fowlpox virus (“F”) components, thus taking advantage of a heterologous prime-boost strategy [25]. Several clinical trials have been conducted using PROSTVAC, including a phase 2 randomized, double-blind placebo-controlled study of PROSTVAC in combination with granulocyte-macrophage colony-stimulating factor (GM-CSF) in patients with mCRPC, which, similar to sipuleucel-T, showed no improvement in their primary endpoint of PFS, but did show a significant OS benefit, compared with placebo (26.2 versus 16.3 months, respectively; stratified log-rank p = 0.0019) [26, 27]. Based on this, a large phase 3 placebo-controlled clinical trial of PROSTVAC combined with GM-CSF was subsequently conducted to confirm the findings of the phase 2 trial [28•]. A total of 432 patients received vaccine-based therapy, and 433 patients were assigned to the placebo arm. The primary endpoint of the study was overall survival (OS), and at the third interim analysis, the trial was stopped early due to futility. No OS benefit was observed, and patients on the vaccine arm also had no improvement in other clinical outcome parameters of interest, including radiographic progression, pain progression, or chemotherapy initiation. The authors hypothesize that the earlier phase 2 trial may have generated a false positive signal due to being underpowered for OS evaluation, or that survival may have been equilibrated due the interim approval of multiple systemic chemotherapy and androgen receptor pathway therapies associated with survival benefit.
Checkpoint Inhibitor Immunotherapy
CTLA-4 inhibitor monotherapy has been evaluated in patients with advanced prostate cancer in two large, randomized, phase III clinical trials. Kwon et al. conducted a trial of 799 patients with metastatic castration-resistant prostate cancer with bone metastases who had progressed on docetaxel chemotherapy [12•]. Patients received directed radiation to 8 Gy to an osseous metastasis and were randomized 1:1 to then receive either ipilimumab 10 mg/kg IV or placebo every 3 weeks. The primary endpoint was overall survival (OS), and there was no statistically significant difference between the groups; the median OS was 11.2 months in the ipilimumab arm and 10.0 months in the placebo arm (HR 0.85, 0.72–1.00, p = 0.053). However, a post hoc assessment demonstrated that patients with more favorable prognostic features, including an alkaline phosphatase level < 1.5 the upper limit of normal, hemoglobin concentration of ≥ 11 g/dL, and absence of visceral metastases, had a prolonged survival of 22.7 months with ipilimumab compared with 15.8 months with placebo (HR = 0.62, 95% CI 045–0.86, p = 0.0038). A subsequent double-blind phase III trial by Beer et al. randomized 598 patients with chemotherapy-naive metastatic castration-resistant prostate cancer in a 2:1 fashion to ipilimumab 10 mg/kg versus placebo [11•]. As in the preceding study, the primary endpoint of overall survival was again not met, but there was a longer progression free survival (PFS) among ipilimumab-treated patients compared with the placebo arm (5.6 months versus 3.8 months, HR = 0.67, 95.87% CI 055–0.81). The ipilimumab group also demonstrated a higher PSA response rate (23% versus 8%), suggesting a population of patients more likely to respond to immunotherapy, but with overall disappointing response rates.
KEYNOTE-028 (NCT02054806) was a phase Ib trial of pembrolizumab, an anti-PD-1 checkpoint inhibitor, in patients with locally advanced or metastatic solid malignancies. This study included 23 patients with castration-resistant metastatic prostate cancer, a RECIST 1.1. measurable lesion, and PD-L1 expression in ≥ 1% tumor or stromal cells. Patients received pembrolizumab 10 mg/kg every 2 weeks until disease progression or unacceptable toxicity. The objective response rate (ORR) was 17.4%, with 4 of 23 patients achieving a partial response [29].
The preceding modest, and largely negative, efficacy data on vaccine-based therapies and single-agent checkpoint immunotherapy in mCRPC underscore the need for novel and combination approaches in this disease space.
Future Approaches: Combination Vaccine and Checkpoint Inhibitor Trials, Molecularly Driven ApproachesVaccine Combination Therapy
-
1.
Vaccine and cytokine combination therapy
Given the relatively limited efficacy of vaccine monotherapy in prostate cancer to date, one emerging therapeutic approach is combining vaccine and cytokine therapy. IL-7 is a homeostatic growth factor for T cells and is capable of inducing proliferation, maintaining T cell responsiveness, and preventing and reversing T cell anergy [30]. A phase 2 randomized, controlled clinical trial is currently underway in which patients with asymptomatic or minimally symptomatic mCRPC were randomized to receive CYT107, a recombinant glycosylated human interleukin-7, after standard sipuleucel-T administration (NCT01881867). The primary objective of this study is to determine whether CYT107 administration increases the vaccine-induced antigen-specific T cell immune response to the sipuleucel-T fusion protein vaccine construct PAP-GM-CSF (PA2024). Patients received CYT107 therapy within 3–7 days of completion of sipuleucel-T therapy, compared with control (no CYT107). This trial has completed accrual and preliminary results presented show CYT107 can induce significant expansion of T cells compared with controls (Pachynski et al. SITC Annual Meeting 2018).
Such combination trials are important steps in improving the efficacy of this already FDA approved therapeutic vaccine; sipuleucel-T has the potential to serve as an important backbone in prostate cancer immunotherapy studies.
-
2.
Vaccine and checkpoint inhibitor combination therapy
Another vaccine-based strategy is centered around the use of attenuated bacterial strains such as Listeria monocytogenes (Lm) as an antigen delivery vector. Listeria monocytogenes (Lm)-listeriolysin O (LLO) therapies can induce antigen-specific T cell responses, and have shown significant anti-tumor efficacy in prostate cancer preclinical models [31, 32]. ADXS31-142 is a live attenuated Lm-LLO which targets PSA and secretes an antigen-adjuvant fusion protein [33]. Preclinical murine models have demonstrated that Lm-LLO in conjunction with an anti-PD-1 antibody inhibits the PD-1/PD-L-1 interaction, resulting in inhibition of tumor growth and prolonged survival in treated animals [34]. This approach is currently being evaluated in the phase 1/2 clinical trial KEYNOTE-046, in which patients with mCRPC are either treated with ADXS310142 alone or in combination with pembrolizumab (NCT02325557). The primary endpoint of the phase 1 portion of the trial is safety as measured in the frequency of adverse events; the secondary outcome measure is progression-free survival per RECIST 1.1. Preliminary results were recently presented, showing that 2 (14%) patients receiving monotherapy and 16 (43%) patients receiving combination therapy had a decreased PSA post-baseline, with 8 (22%) of the combination patients having PSA reductions of ≥ 50% from baseline. These data support the added benefit of checkpoint inhibition in the setting of a vaccine strategy.
Other vaccine combination approaches employ DNA vaccines in conjunction with checkpoint inhibitor immunotherapy. One vaccine-based approach is being tested in an ongoing phase I clinical trial (NCT02616185) utilizing escalating doses of a vaccine-based immunotherapy regimen (VBIR, Pfizer). Planned accrual is for 133 patients who have either a prior history of prostate cancer and hormone-sensitive biochemical relapse, those with disease progression during post-surgical castration or ongoing androgen deprivation therapy (pre-secondary hormone CRPC), or who have documented disease progression after secondary hormone therapy such as abiraterone acetate or enzalutamide (post-secondary hormone CRPC). Patients will receive three different biologic agents administered at various dosing intervals: an adenoviral vaccine (PF-06755992) on day 1 of each cycle, a DNA plasmid vaccine (PF-06755990) on days 27, 57, and 85 via a TDS-IM electroporation device, and an anti-PD1 monoclonal antibody (PF-06801591) every 28 days. Concurrently, patients will receive oral sunitinib, with the maximum tolerated dose (MTD) identified during the course of the study, as well as tremelimumab, an anti-CTLA-4 monoclonal antibody, every 28 days. The primary outcome measure is incidence and grade of treatment-emergent adverse events, including dose-limiting toxicities (DLTs). Multiple secondary outcome measures are planned, including immune response to selected prostate cancer tumor antigens and antibody response to PSMA antigen. Study completion is anticipated in late 2021.
In pre-clinical studies, McNeel and colleagues showed that vaccination using a DNA vector platform resulted in increased PD-1 expression on antigen-specific CD8+ T cells which limited anti-tumor efficacy. They were able to successfully reverse this using concomitant PD-1 blockade resulting in significantly improved anti-tumor responses [35, 36]. A subsequent pilot clinical trial evaluated sequential or concurrent administration of a DNA-based vaccine and anti-PD-1 checkpoint inhibitor immunotherapy with pembrolizumab in patients (n = 26) with mCRPC [37]. Overall, the concurrent treatment was tolerated well with no unanticipated adverse events. Interestingly, 8/13 (62%) of patients treated concurrently, while only 1/12 (8%, p = 0.01) of patients treated sequentially, experienced PSA declines from baseline. Both groups had increases in PAP-specific interferon gamma or granzyme B–secreting T cells, but PSA declines were associated with the development of PAP-specific Th1-biased T cell immunity and CD8+ T cell infiltration in metastatic tumor biopsy specimens in those patients who received concurrent treatment. This suggests the efficacy of a combinatorial approach utilizing concurrent checkpoint blockade may be successful in increasing immune infiltration in an otherwise immunologically “cold” tumor.
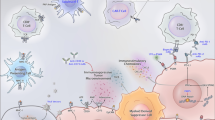
To this end, an ongoing clinical trial at our institution is evaluating a personalized neoantigen DNA vaccine in combination with ipilimumab/nivolumab and PROSTVAC in metastatic prostate cancer (NCT03532217) (see schema, Fig. 1). Of note, given the previously discussed negative phase III trial of single agent PROSTVAC in patients with mCRPC, this study seeks to evaluate the immune responses, safety/tolerability, and efficacy of a vaccine-based combination immunotherapeutic approach in patients with metastatic hormone-sensitive prostate cancer (mHSPC)—thus moving combination immunotherapy earlier in the disease state. Patients with untreated high-volume metastatic disease receive planned standard of care docetaxel chemotherapy dosed every 3 weeks for a planned 6 cycles, along with continuous standard androgen deprivation therapy (ADT). After completion of chemotherapy, patients will receive nivolumab/ipilimumab plus PROSTVAC followed by a personalized neoantigen DNA vaccine. Within 60 days after the last dose of docetaxel, patients will start a priming dose of PROSTVAC-V as a single agent, and subsequent doses of PROSTVAC-F in combination with ipilimumab (1 mg/kg every 3 weeks for 2 doses), and nivolumab (3 mg/kg every 3 weeks for 6 doses) over a course of approximately 17 weeks. Patients will then receive a personalized neoantigen DNA vaccine based off of their metastatic biopsy for a total of 6 treatments every 28 days along with nivolumab 480 mg IV. The neoantigen vaccine is delivered via electroporation, which has been shown to enhance vaccine delivery and responses [38]. The primary efficacy endpoint of the trial is failure-free survival (FFS) and milestone survival of the combination therapy.
A pilot trial of neoantigen DNA vaccine in combination with nivolumab/ipilimumab and Prostvac in metastatic hormone-sensitive prostate cancer (NCT03532217). Patients who successfully complete an initial course of docetaxel chemotherapy for mHSPC will then receive the combination of Prostvac-VF plus checkpoint inhibitors (“Treatment A”), as indicated. A personalized neoantigen DNA vaccine is produced during that time, and subsequently administered with continued anti-PD-1 checkpoint blockade (“Treatment B”)
Ongoing Checkpoint Inhibitor Immunotherapy Trials
Several checkpoint inhibitor clinical trials are aiming to sub-stratify patients by parameters such as disease burden, prior therapy, and other potential biomarkers of response such as PD-L1 status and tumor mutation burden (TMB), with completed and ongoing trials summarized in Table 1
. KEYNOTE-199 (NCT02787005) is a phase II clinical trial of pembrolizumab monotherapy in patients with docetaxel-refractory mCRPC. Patients were stratified into cohorts according to PD-L1 status and presence or absence of RECIST-measurable disease. Cohort 1–enrolled patients with measurable disease and PD-L1 positive tumors; cohort 2–enrolled patients with measurable disease and PD-L1 negative tumors, and cohort 3–enrolled patients with non-measurable, bone-predominant disease. Initial data for cohorts 1–3 was reported in 2018, with an overall disease control rate (CR + PR + SD) across cohorts of 11% [39]. CheckMate-650 is evaluating combination anti-CTLA-4 and anti-PD-1 immunotherapy in patients with mCRPC and progression on second generation hormone therapy. Patients receive ipilimumab 3 mg/kg IV and nivolumab 1 mg/kg IV every 3 weeks for 4 doses followed by nivolumab 480 mg IV every 4 weeks [40]. A pre-planned interim analysis after 78 patients had a minimum of 6 months of follow-up demonstrated an ORR of 26% in chemotherapy-naïve patients and 10% in patients who had received prior chemotherapy. ORR rates were higher in patients with PD-L1 ≥ 1%, DNA damage repair (DDR), homologous recombination deficiency (HRD), or a high tumor mutation burden (TMB), defined as greater than 74.5 mutations. Importantly, high TMB (above the median) was associated with significantly improved rPFS vs low TMB (below the median) (p < 0.0001) [40], suggesting even within prostate cancer, mutational burden status likely impacts responses to checkpoint inhibitors. The PERSEUS1 trial (NCT03506997) is a phase II, single-arm study of pembrolizumab in patients with mCRPC with disease progression on at least one prior line of therapy in the metastatic castration-resistant setting, either chemotherapy or hormonal therapy. Patients in this trial must have a high mutational load (> 11 mutations per targeted panel), and/or a DNA repair defect that can increase mutational load such as dMMR/MSI-H. The primary endpoint is objective response rate (ORR), PSA decline of ≥ 50%, and circulating tumor cell (CTC) count conversion from > 5 to < 5.
There are multiple ongoing clinical trials assessing checkpoint inhibitor immunotherapy in conjunction with anti-androgen therapy, in particular in the setting of prior resistance to androgen-receptor pathway inhibitors. Previously published data has suggested an increased likelihood of response to checkpoint inhibitor immunotherapy in patients with prior enzalutamide resistance [41, 42]. A phase 2 clinical trial (NCT02312557) is currently underway in patients with mCRPC who previously progressed on enzalutamide therapy. Fifty-eight patients will be enrolled and will receive ongoing enzalutamide along with pembrolizumab. The primary endpoint of this study is PSA ≥ 50% response rate. An ongoing multi-center phase II clinical trial (NCT03248570) is enrolling men with mCRPC who received either abiraterone acetate and/or enzalutamide and will receive pembrolizumab but will be stratified to two treatment arms: those that are DNA damage repair proficient or deficient, respectively. The primary endpoint for this trial is the objective response rate in the DNA damage repair proficient and deficient groups.
Cohort C of KEYNOTE-365 (NCT02861573) is evaluating pembrolizumab plus enzalutamide in abiraterone-pretreated patients with metastatic castrate-resistant prostate cancer (mCRPC) [43]. There are several ongoing phase 3 clinical trials evaluation combinations of androgen receptor pathway inhibitors and immunotherapy that should add important data to the current therapeutic landscape in advanced prostate cancer. IMbassador250 (NCT03016312) is a phase 3 clinical trial of enzalutamide with or without atezolizumab in mCRPC patients who have progressed on an androgen synthesis inhibitor and are unable to receive taxane chemotherapy. KEYNOTE-641 (NCT03834493) is a phase 3, randomized, double blind clinical trial of pembrolizumab and enzalutamide versus placebo and enzalutamide in patients with mCRPC.
Despite the overall relatively low response rates in some of the early published data from these clinical trials, it is nonetheless encouraging that subpopulations of patients have demonstrated a response to checkpoint inhibitor immunotherapy, suggesting that appropriate patient selection may be a key factor in future trial design. Moreover, appropriate biomarker selection to screen to potential responsiveness to checkpoint inhibitor therapy is still lacking in prostate cancer. In one series of immunohistochemical analysis of PD-L1 in primary prostate cancers, 52.2 to 61.7% demonstrated moderate to high PD-L1 expression, a finding that is notably discordant from published clinical trial data to date [44]. Thus, additional strategies to identify robust predictive biomarkers of response to immunotherapy are needed in prostate cancer.
Molecular Selection to Optimize Response to Checkpoint Inhibitor Immunotherapy
While one avenue to improving the likelihood of response to checkpoint inhibitor immunotherapy in patients with prostate cancer is developing combination treatments with vaccines or implementing combination immunotherapy treatments, another important consideration is patient selection. Previous clinical trials have demonstrated relatively low response rates in an unselected mCRPC population, but within these cohorts there nonetheless appear to be patient subsets with a higher response rate to immunotherapy. With the advent of more widespread genomic analysis of advanced prostate cancer, tailoring therapies to genomically selected prostate cancer sub-populations are now feasible [45••, 46].
Pembrolizumab was FDA approved in 2017 as second-line therapy in a tumor-agnostic fashion for patients with advanced malignancies who harbor DNA mismatch repair (MMR) deficiencies or are microsatellite instability-high (MSI-H). dMMR/MSI-H aberrations have been identified in about 2–3% of patients with advanced prostate cancer [45••, 47, 48]. Antonarakis et al. reported four dMMR mCRPC patients who received anti-PD-1 checkpoint inhibitor therapy with nivolumab or pembrolizumab in the fourth-line setting or beyond, and two patients attained a > 50% reduction in PSA; three patients had an objective radiographic response in soft tissue metastases [47]. In a retrospective series by Abida et al., 11 patients with dMMR/MSI-H prostate tumors received anti-PD-1 or anti-PD-L1 immunotherapy, and six of these patients achieved a > 50% PSA decline [49]. Despite the low incidence of mismatch repair deficiencies in prostate cancer, the promising response rates in these studies highlight the importance of rigorous and ubiquitous molecular evaluation of patients with advanced prostate cancer.
Prostate cancer patients whose tumors harbor DNA repair defects appear to have a consistently higher response rate to checkpoint inhibitor immunotherapy compared with unselected prostate cancer patients. In a phase II trial, patients with mCRPC and disease progression on abiraterone and/or enzalutamide were treated with the combination of the anti-PD-1 antibody durvalumab and the PARP inhibitor olaparib [50]. Nine of 15 patients (53%) had a ≥ 50% PSA decline; of note, four responders had germline alterations in DNA damage repair (DDR) genes, including BRCA2, and two additional responders had somatic BRCA2 alterations. In another phase II clinical trial, 15 patients with mCRPC and AR-V7 positive circulating tumor cells (CTCs) were treated with combination therapy with ipilimumab and nivolumab [51]. Six of the 15 patients (40%) were found to have deleterious mutations in DNA repair genes, both somatic or germline. There was a trend toward improved PSA response rates and ORR rates in the patients with DDR mutations.
Metastatic prostate cancers harboring loss of CDK12 have been identified at a frequency of about 3–7% [45••, 46, 52•]. In mCRPC, CDK12 loss results in extensive focal tandem duplications throughout the genome, resulting in a high neoantigen burden [52•]. Pilot clinical data of patients with CDK12 loss who were treated with anti-PD-1 checkpoint inhibitor immunotherapy demonstrated robust PSA decline and radiographic responses in 2 of 4 patients [52•]. A multi-center, phase II clinical trial of patients with metastatic cancers, including mCRPC, harboring CDK12 alterations is currently underway (NCT03570619). Patients are treated with combination checkpoint inhibitor immunotherapy with ipilimumab 1 mg/kg IV and nivolumab 3 mg/kg IV every 3 weeks for up to 4 doses, followed by nivolumab 480 mg IV every 4 weeks. The primary objective is the overall response rate in patients with mCRPC; response is defined as 50% decline in PSA as per PCWG3 criteria. Given the multiple antecedent clinical trials with checkpoint inhibitor immunotherapy in unselected mCRPC patients, this study will provide valuable insight into genomically selecting prostate cancer patients for immunotherapy trials.
Conclusions
Despite multiple promising advances—and a current FDA-approved treatment—in the development of prostate cancer immunotherapies, substantial progress in improving the efficacy in prostate cancer patients is still lacking. Nonetheless, it is promising that as further innovative clinical trials are developed, including advances in CAR-T cell therapy in prostate cancer through PSMA-based designs (NCT03089203), more effective immunotherapeutics for prostate cancer continue to become feasible. There are multiple factors contributing to the discrepancy in the abundance of ongoing clinical trials and paucity of resultant practice-changing results. It is evident that our understanding of the heterogeneity of prostate tumors, and the complexities of the prostate cancer tumor immune microenvironment (TIME), are still incomplete [53, 54]. The relative importance and contribution of tumor-infiltrating leukocytes (whether anti- or pro-tumor), interferon gamma and other cytokine responses, and tumor volume all remain incompletely characterized in prostate cancer. While optimal approaches and combinations are still being developed and tested, it is now clear from past clinical trials with a variety of immunotherapies that a “single agent” approach will likely be insufficient to see real efficacy gains in mCRPC. For example, while single agent checkpoint inhibitors have garnered robust responses and FDA approvals across several tumor types, response rates of ~ 10% are seen in mCRPC. Whether due to differences in mutational burden and neoantigen expression or the immune milieu within the tumor microenvironment, prostate cancer appears to require additional immunomodulation. Combinatorial approaches, many of which have been discussed here, will be the future of prostate cancer immunotherapy—though, rational combinations must be pursued based on solid prostate tumor immunobiology. Whether off-the-shelf or personalized vaccines, combinations with cytokines or checkpoint inhibitors, or incorporating additional modalities such as radiotherapy, successful treatment regimens will have to take a multi-pronged approach. While the growing number of immunotherapy clinical trials in prostate cancer is encouraging, the relative paucity of robust response data and the limited drug approvals for prostate cancer over the last decade serve as a stark reminder of how much more work there is to be done in this field.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67(1):7–30.
Kelly SP, Anderson WF, Rosenberg PS, Cook MB. Past, current, and future incidence rates and burden of metastatic prostate cancer in the United States. Eur Urol Focus. 2018;4(1):121–7.
Hurwitz AA, Foster BA, Kwon ED, Truong T, Choi EM, Greenberg NM, et al. Combination immunotherapy of primary prostate cancer in a transgenic mouse model using CTLA-4 blockade. Cancer Res. 2000;60(9):2444–8.
van Elsas A, Hurwitz AA, Allison JP. Combination immunotherapy of B16 melanoma using anti- cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) and granulocyte/macrophage colony-stimulating factor (GM-CSF)-producing vaccines induces rejection of subcutaneous and metastatic tumors accompanied by autoimmune depigmentation. J Exp Med. 1999;190(3):355–66.
Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–23.
Robert C, Schachter J, Long GV, Arance A, Grob JJ, Mortier L, et al. Pembrolizumab versus Ipilimumab in advanced melanoma. N Engl J Med. 2015;372(26):2521–32.
Gandhi L, Rodriguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med. 2018;378(22):2078–92.
Paz-Ares L, Luft A, Vicente D, Tafreshi A, Gumus M, Mazieres J, et al. Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N Engl J Med. 2018;379(21):2040–51.
Mehra R, Seiwert TY, Gupta S, Weiss J, Gluck I, Eder JP, et al. Efficacy and safety of pembrolizumab in recurrent/metastatic head and neck squamous cell carcinoma: pooled analyses after long-term follow- up in KEYNOTE-012. Br J Cancer. 2018;119(2):153–9.
Balar AV, Castellano D, O'Donnell PH, Grivas P, Vuky J, Powles T, et al. First-line pembrolizumab in cisplatin-ineligible patients with locally advanced and unresectable or metastatic urothelial cancer (KEYNOTE-052): a multicentre, single-arm, phase 2 study. Lancet Oncol. 2017;18(11):1483–92.
• Beer TM, Kwon ED, Drake CG, Fizazi K, Logothetis C, Gravis G, et al. Randomized, double-blind, phase iii trial of ipilimumab versus placebo in asymptomatic or minimally symptomatic patients with metastatic chemotherapy-naive castration-resistant prostate cancer. J Clin Oncol. 2017;35(1):40–7 Randomized, placebo-controlled phase III clinical trial of 598 patients with asymptomatic or minimally symptomatic chemotherapy-naïve mCRPC. Patients were randomized 2:1 to receive ipilimumab 10 mg/kg IV every 3 weeks or placebo. Primary endpoint of overall survival was not met.
• Kwon ED, Drake CG, Scher HI, Fizazi K, Bossi A, van den Eertwegh AJ, et al. Ipilimumab versus placebo after radiotherapy in patients with metastatic castration-resistant prostate cancer that had progressed after docetaxel chemotherapy (CA184-043): a multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol. 2014;15(7):700–12 Randomized, placebo-controlled phase III clinical trial of 799 mCRPC patients with progression on docetaxel chemotherapy. Patients received 8 Gy radiation directed to an osseous metastasis followed by either ipilimumab 10 mg/kg IV every 3 weeks or placebo. Primary endpoint of overall survival was not met.
Lawrence MS, Stojanov P, Polak P, Kryukov GV, Cibulskis K, Sivachenko A, et al. Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature. 2013;499(7457):214–8.
Danaher P, Warren S, Lu R, Samayoa J, Sullivan A, Pekker I, et al. Pan-cancer adaptive immune resistance as defined by the tumor inflammation signature (TIS): results from The Cancer Genome Atlas (TCGA). J Immunother Cancer. 2018;6(1):63.
Small EJ, Schellhammer PF, Higano CS, Redfern CH, Nemunaitis JJ, Valone FH, et al. Placebo- controlled phase III trial of immunologic therapy with sipuleucel-T (APC8015) in patients with metastatic, asymptomatic hormone refractory prostate cancer. J Clin Oncol. 2006;24(19):3089–94.
Higano CS, Schellhammer PF, Small EJ, Burch PA, Nemunaitis J, Yuh L, et al. Integrated data from 2 randomized, double-blind, placebo-controlled, phase 3 trials of active cellular immunotherapy with sipuleucel-T in advanced prostate cancer. Cancer. 2009;115(16):3670–9.
•• Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363(5):411–22 Randomized, placebo-controlled phase III clinical trial of 512 patients with asymptomatic or minimally symptomatic mCRPC. Patients were randomized 2:1 to receive sipuleucel-T or placebo. The study met its primary endpoint of overall survival, with a median survival of 25.8 months in the sipuleucel-T arm and 21.7 months in the control arm, for a benefit of 4.1 months. Sipuleucel-T was FDA approved in 2010.
Schellhammer PF, Chodak G, Whitmore JB, Sims R, Frohlich MW, Kantoff PW. Lower baseline prostate-specific antigen is associated with a greater overall survival benefit from sipuleucel-T in the immunotherapy for prostate adenocarcinoma treatment (IMPACT) trial. Urology. 2013;81(6):1297–302.
Holl EK, McNamara MA, Healy P, Anand M, Concepcion RS, Breland CD, et al. Prolonged PSA stabilization and overall survival following sipuleucel-T monotherapy in metastatic castration-resistant prostate cancer patients. Prostate Cancer Prostatic Dis. 2019.
Sartor AO, Armstrong A, Ahaghotu C, McLeod D, Cooperberg M, Penson D, et al. PD24-12 overall survival analysis of African American and Caucasian patients receiving sipuleucel-T: preliminary data from the proceed registry. J Urol. 2017;197(4S):e456–e7.
Twardowski P, Wong JYC, Pal SK, Maughan BL, Frankel PH, Franklin K, et al. Randomized phase II trial of sipuleucel-T immunotherapy preceded by sensitizing radiation therapy and sipuleucel-T alone in patients with metastatic castrate resistant prostate cancer. Cancer Treat Res Commun. 2019;19:100116.
Fong L, Carroll P, Weinberg V, Chan S, Lewis J, Corman J, et al. Activated lymphocyte recruitment into the tumor microenvironment following preoperative sipuleucel-T for localized prostate cancer. J Natl Cancer Inst. 2014;106(11).
Caram MEV, Ross R, Lin P, Mukherjee B. Factors associated with use of sipuleucel-T to treat patients with advanced prostate cancer. JAMA Netw Open. 2019;2(4):e192589.
Simons JW, Carducci MA, Mikhak B, Lim M, Biedrzycki B, Borellini F, et al. Phase I/II trial of an allogeneic cellular immunotherapy in hormone-naive prostate cancer. Clin Cancer Res. 2006;12(11 Pt 1):3394–401.
Madan RA, Arlen PM, Mohebtash M, Hodge JW, Gulley JL. Prostvac-VF: a vector-based vaccine targeting PSA in prostate cancer. Expert Opin Investig Drugs. 2009;18(7):1001–11.
Kantoff PW, Schuetz TJ, Blumenstein BA, Glode LM, Bilhartz DL, Wyand M, et al. Overall survival analysis of a phase II randomized controlled trial of a Poxviral-based PSA-targeted immunotherapy in metastatic castration-resistant prostate cancer. J Clin Oncol. 2010;28(7):1099–105.
Kantoff PWGJ, Pico-Navarro C. Revised overall survival analysis of a phase II, randomized, double- blind, controlled study of PROSTVAC in men with metastatic castration-resistant prostate cancer. J Clin Oncol. 2017;35:124–5.
• Gulley JL, Borre M, Vogelzang NJ, Ng S, Agarwal N, Parker CC, et al. Phase III Trial of PROSTVAC in asymptomatic or minimally symptomatic metastatic castration-resistant prostate cancer. J Clin Oncol. 2019;37(13):1051–61 Randomized, placebo-controlled phase III clinical trial of 864 patients with mCRPC randomized to receive PROSTVAC + GM-CSF or placebo. The primary endpoint was overall survival, and the study was halted prematurely at the third interim analysis due to futility.
Hansen AR, Massard C, Ott PA, Haas NB, Lopez JS, Ejadi S, et al. Pembrolizumab for advanced prostate adenocarcinoma: findings of the KEYNOTE-028 study. Ann Oncol. 2018;29(8):1807–13.
Mackall CL, Fry TJ, Gress RE. Harnessing the biology of IL-7 for therapeutic application. Nat Rev Immunol. 2011;11(5):330–42.
Johnson LE, Brockstedt D, Leong M, Lauer P, Theisen E, Sauer JD, et al. Heterologous vaccination targeting prostatic acid phosphatase (PAP) using DNA and Listeria vaccines elicits superior anti-tumor immunity dependent on CD4+ T cells elicited by DNA priming. Oncoimmunology. 2018;7(8):e1456603.
Hannan R, Zhang H, Wallecha A, Singh R, Liu L, Cohen P, et al. Combined immunotherapy with Listeria monocytogenes-based PSA vaccine and radiation therapy leads to a therapeutic response in a murine model of prostate cancer. Cancer Immunol Immunother. 2012;61(12):2227–38.
NBea H. Phase I-II study of ADXS31-142 alone and in combination with pembrolizumab in patients with previously treated metastatic castration-resistant prostate cancer (mCRPC): the KEYNOTE- 046 trial. J Immunother Cancer. 2015;3(Suppl2):P153.
Mkrtichyan M, Chong N, Abu Eid R, Wallecha A, Singh R, Rothman J, et al. Anti-PD-1 antibody significantly increases therapeutic efficacy of Listeria monocytogenes (Lm)-LLO immunotherapy. J Immunother Cancer. 2013;1:15.
Rekoske BT, Smith HA, Olson BM, Maricque BB, McNeel DG. PD-1 or PD-L1 blockade restores antitumor efficacy following SSX2 epitope-modified DNA vaccine immunization. Cancer Immunol Res. 2015;3(8):946–55.
Zahm CD, Colluru VT, McNeel DG. Vaccination with high-affinity epitopes impairs antitumor efficacy by increasing PD-1 expression on CD8(+) T cells. Cancer immunology research. 2017;5(8):630–41.
McNeel DG, Eickhoff JC, Wargowski E, Zahm C, Staab MJ, Straus J, et al. Concurrent, but not sequential, PD-1 blockade with a DNA vaccine elicits anti-tumor responses in patients with metastatic, castration-resistant prostate cancer. Oncotarget. 2018;9(39):25586–96.
Lambricht L, Lopes A, Kos S, Sersa G, Preat V, Vandermeulen G. Clinical potential of electroporation for gene therapy and DNA vaccine delivery. Expert Opin Drug Deliv. 2016;13(2):295–310.
de Bono JS. KEYNOTE-199: Pembrolizumab (pembro) for docetaxel-refractory metastatic castration-resistant prostate cancer (mCRPC). Journal of Clinical Oncology. 2018;36(15):suppl.5007.
Padmanee S, Russel KP, Vivek N, Aude F, Gwenaelle G, Matt DG, et al. Initial results from a phase II study of nivolumab (NIVO) plus ipilimumab (IPI) for the treatment of metastatic castration-resistant prostate cancer (mCRPC; CheckMate 650). Journal of Clinical Oncology. 2019;37(7):suppl.142.
Graff JN, Alumkal JJ, Drake CG, Thomas GV, Redmond WL, Farhad M, et al. Early evidence of anti- PD-1 activity in enzalutamide-resistant prostate cancer. Oncotarget. 2016;7(33):52810–7.
Bishop JL, Sio A, Angeles A, Roberts ME, Azad AA, Chi KN, et al. PD-L1 is highly expressed in enzalutamide resistant prostate cancer. Oncotarget. 2015;6(1):234–42.
Fong P. Keynote-365 cohort C: Pembrolizumab (pembro) plus enzalutamide (enza) in abiraterone (abi)-pretreated patients (pts) with metastatic castrate resistant prostate cance (mCRPC). Journal of Clinical Oncology. 2019;37(7):suppl (March 1 2019)):171.
Gevensleben H, Dietrich D, Golletz C, Steiner S, Jung M, Thiesler T, et al. The immune checkpoint regulator PD-L1 is highly expressed in aggressive primary prostate cancer. Clin Cancer Res. 2016;22(8):1969–77.
•• Robinson D, Van Allen EM, Wu YM, Schultz N, Lonigro RJ, Mosquera JM, et al. Integrative clinical genomics of advanced prostate cancer. Cell. 2015;162(2):454 Comprehensive analysis of the genomic landscape of 150 patients with mCRPC. Aberrations of AR , ETS genes, TP53 and PTEN were most frequent.
Quigley DA, Dang HX, Zhao SG, Lloyd P, Aggarwal R, Alumkal JJ, et al. Genomic hallmarks and structural variation in metastatic prostate cancer. Cell. 2018;175(3):889.
Antonarakis ES, Shaukat F, Isaacsson Velho P, Kaur H, Shenderov E, Pardoll DM, et al. Clinical features and therapeutic outcomes in men with advanced prostate cancer and DNA mismatch repair gene mutations. Eur Urol. 2019;75(3):378–82.
Le DT, Durham JN, Smith KN, Wang H, Bartlett BR, Aulakh LK, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357(6349):409–13.
Abida W, Cheng ML, Armenia J, Middha S, Autio KA, Vargas HA, et al. Analysis of the Prevalence of Microsatellite Instability in Prostate Cancer and Response to Immune Checkpoint Blockade. JAMA Oncol. 2019;5(4):471-8.
Karzai F, VanderWeele D, Madan RA, Owens H, Cordes LM, Hankin A, et al. Activity of durvalumab plus olaparib in metastatic castration-resistant prostate cancer in men with and without DNA damage repair mutations. J Immunother Cancer. 2018;6(1):141.
Boudadi K, Suzman DL, Anagnostou V, Fu W, Luber B, Wang H, et al. Ipilimumab plus nivolumab and DNA-repair defects in AR-V7-expressing metastatic prostate cancer. Oncotarget. 2018;9(47):28561–71.
• Wu YM, Cieslik M, Lonigro RJ, Vats P, Reimers MA, Cao X, et al. Inactivation of CDK12 delineates a distinct immunogenic class of advanced prostate cancer. Cell. 2018;173(7):1770–82 e14 CDK12 loss has been identified as a novel subtype of mCRPC, characterized by focal tandem duplications and increased gene fusions.
Massari F, Ciccarese C, Calio A, Munari E, Cima L, Porcaro AB, et al. Magnitude of PD-1, PD-L1 and T lymphocyte expression on tissue from castration-resistant prostate adenocarcinoma: an exploratory analysis. Target Oncol. 2016;11(3):345–51.
Vitkin N, Nersesian S, Siemens DR, Koti M. The tumor immune contexture of prostate cancer. Front Immunol. 2019;10:603.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Melissa A. Reimers and Kathryn Slane each declares no potential conflicts of interest.
Russell K. Pachynski reports personal fees from Sanofi, EMD Serono, Pfizer, Jounce, Dendreon, Bayer, Genomic Health, Merck, Genentech/Roche, and AstraZeneca; research collaboration/support from Janssen; and institutional support from Genentech/Roche.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Prostate Cancer
Rights and permissions
About this article
Cite this article
Reimers, M.A., Slane, K.E. & Pachynski, R.K. Immunotherapy in Metastatic Castration-Resistant Prostate Cancer: Past and Future Strategies for Optimization. Curr Urol Rep 20, 64 (2019). https://doi.org/10.1007/s11934-019-0931-3
Published:
DOI: https://doi.org/10.1007/s11934-019-0931-3