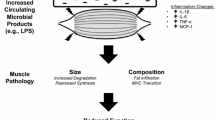
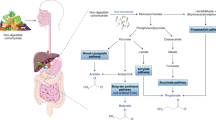
Abstract
Purpose of Review
This review aims to summarize the recent findings about the contribution of the gut microbiome to muscle pathophysiology and discuss molecular pathways that may be involved in such process. Related findings in the context of cancer cachexia are outlined.
Recent Findings
Many bacterial metabolites have been reported to exert a beneficial or detrimental impact on muscle physiology. Most of the evidence concentrates on short-chain fatty acids (SCFAs), with an emerging role for bile acids, bacterial amino acid metabolites (bAAms), and bacterial polyphenol metabolites. Other molecular players worth considering include cytokines, hormones, lipopolysaccharides, and quorum sensing molecules.
Summary
The current literature clearly establishes the ability for the gut microbiome to modulate muscle function and mass. The understanding of the mechanisms underlying this gut-muscle axis may lead to the delivery of novel therapeutic tools to tackle muscle wasting in cancer cachexia, chronic kidney disease, liver fibrosis, and age-related sarcopenia.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Frontera WR, Ochala J. Skeletal muscle: a brief review of structure and function. Calcif Tissue Int. 2015;96(3):183–95.
Sartori R, Romanello V, Sandri M. Mechanisms of muscle atrophy and hypertrophy: implications in health and disease. Nat Commun. 2021;12(1):330. A review summarizing the main molecular pathways involved in the regulation of muscle.
Lee YH, Kim SU, Song K, Park JY, Kim DY, Ahn SH, Lee BW, Kang ES, Cha BS, Han KH. Sarcopenia is associated with significant liver fibrosis independently of obesity and insulin resistance in nonalcoholic fatty liver disease: Nationwide surveys (KNHANES 2008-2011). Hepatology. 2016;63(3):776–86.
Prado CM, Purcell SA, Laviano A. Nutrition interventions to treat low muscle mass in cancer. J Cachexia Sarcopenia Muscle. 2020;11(2):366–80.
Prado CM, Purcell SA, Alish C, Pereira SL, Deutz NE, Heyland DK, Goodpaster BH, Tappenden KA, Heymsfield SB. Implications of low muscle mass across the continuum of care: a narrative review. Ann Med. 2018;50(8):675–93.
Maddocks M, Hopkinson J, Conibear J, Reeves A, Shaw C, Fearon KC. Practical multimodal care for cancer cachexia. Curr Opin Support Palliat Care. 2016;10(4):298–305.
Potgens SA, Sboarina M, Bindels LB. Polyunsaturated fatty acids, polyphenols, amino acids, prebiotics: can they help to tackle cancer cachexia and related inflammation? Curr Opin Clin Nutr Metab Care. 2018;21(6):458–64.
Bindels LB, Delzenne NM. Muscle wasting: the gut microbiota as a new therapeutic target? Int J Biochem Cell Biol. 2013;45(10):2186–90.
Bindels LB, Thissen JP. Nutrition in cancer patients with cachexia: a role for the gut microbiota? Clin Nutr Exp. 2016;6:74–82.
Ziemons J, Smidt ML, Damink SO, Rensen SS. Gut microbiota and metabolic aspects of cancer cachexia. Best Pract Res Clin Endocrinol Metab. 2021;101508.
Genton L, Cani PD, Schrenzel J. Alterations of gut barrier and gut microbiota in food restriction, food deprivation and protein-energy wasting. Clin Nutr. 2015;34(3):341–9.
Berg G, Rybakova D, Fischer D, Cernava T, Vergès MC, Charles T, et al. Microbiome definition re-visited: old concepts and new challenges. Microbiome. 2020;8(1):103.
Delzenne NM, Knudsen C, Beaumont M, Rodriguez J, Neyrinck AM, Bindels LB. Contribution of the gut microbiota to the regulation of host metabolism and energy balance: a focus on the gut-liver axis. Proc Nutr Soc. 2019:1–10.
Makki K, Deehan EC, Walter J, Backhed F. The impact of dietary fiber on gut microbiota in host health and disease. Cell Host Microbe. 2018;23(6):705–15.
Valdes AM, Walter J, Segal E, Spector TD. Role of the gut microbiota in nutrition and health. BMJ. 2018;361:k2179. A review introducing key concepts in the gut microbiota field, how nutrition impacts the gut microbiota, and mechanisms through which the gut microbiota modulates host health.
Gibson GR, Hutkins R, Sanders ME, Prescott SL, Reimer RA, Salminen SJ, Scott K, Stanton C, Swanson KS, Cani PD, Verbeke K, Reid G. Expert consensus document: the International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat Rev Gastroenterol Hepatol. 2017;14(8):491–502.
Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, Morelli L, Canani RB, Flint HJ, Salminen S, Calder PC, Sanders ME. Expert consensus document: the International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. 2014;11:506–14.
Agus A, Planchais J, Sokol H. Gut microbiota regulation of tryptophan metabolism in health and disease. Cell Host Microbe. 2018;23(6):716–24.
Oliphant K, Allen-Vercoe E. Macronutrient metabolism by the human gut microbiome: major fermentation by-products and their impact on host health. Microbiome. 2019;7(1):91. A review describes in detail macronutrient metabolism by the gut microbiome and how the ensuing metabolites influence human health.
Postler TS, Ghosh S. Understanding the holobiont: how microbial metabolites affect human health and shape the immune system. Cell Metab. 2017;26(1):110–30.
Backhed F, Ding H, Wang T, Hooper LV, Koh GY, Nagy A, et al. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci USA. 2004;101(44):15718–23.
Lahiri S, Kim H, Garcia-Perez I, Reza MM, Martin KA, Kundu P, et al. The gut microbiota influences skeletal muscle mass and function in mice. Sci Transl Med. 2019;11(502). This research article is the first one to provide a detailed characterization of the muscle of germ-free mice, compared to conventional and conventionalized mice, and to establish the beneficial impact of short-chain fatty acids on muscle mass and function.
Nay K, Jollet M, Goustard B, Baati N, Vernus B, Pontones M, et al. Gut bacteria are critical for optimal muscle function: a potential link with glucose homeostasis. Am J Physiol Endocrinol Metab. 2019. This research article contributes significantly to the evidence that gut microbiota modulates muscle function, as established through the administration of a broad spectrum antibiotics cocktail.
Bindels LB, Segura Munoz RR, Gomes-Neto JC, Mutemberezi V, Martinez I, Salazar N, et al. Resistant starch can improve insulin sensitivity independently of the gut microbiota. Microbiome. 2017;5(1):12.
Zarrinpar A, Chaix A, Xu ZZ, Chang MW, Marotz CA, Saghatelian A, Knight R, Panda S. Antibiotic-induced microbiome depletion alters metabolic homeostasis by affecting gut signaling and colonic metabolism. Nat Commun. 2018;9(1):2872.
Huang WC, Chen YH, Chuang HL, Chiu CC, Huang CC. Investigation of the effects of microbiota on exercise physiological adaption, performance, and energy utilization using a gnotobiotic animal model. Front Microbiol. 2019;10:1906.
Lee MC, Hsu YJ, Ho HH, Hsieh SH, Kuo YW, Sung HC, et al. Lactobacillus salivarius subspecies salicinius SA-03 is a new probiotic capable of enhancing exercise performance and decreasing fatigue. Microorganisms. 2020;8(4).
Chen YM, Wei L, Chiu YS, Hsu YJ, Tsai TY, Wang MF, Huang CC. Lactobacillus plantarum TWK10 supplementation improves exercise performance and increases muscle mass in mice. Nutrients. 2016;8(4):205.
Huang WC, Hsu YJ, Huang CC, Liu HC, Lee MC. Exercise training combined with Bifidobacterium longum OLP-01 supplementation improves exercise physiological adaption and performance. Nutrients. 2020;12(4).
Munukka E, Rintala A, Toivonen R, Nylund M, Yang B, Takanen A, Hänninen A, Vuopio J, Huovinen P, Jalkanen S, Pekkala S. Faecalibacterium prausnitzii treatment improves hepatic health and reduces adipose tissue inflammation in high-fat fed mice. ISME J. 2017;11(7):1667–79.
Li G, Jin B, Fan Z. Mechanisms involved in gut microbiota regulation of skeletal muscle. Oxid Med Cell Longev. 2022;2022:2151191–15.
Giron M, Thomas M, Dardevet D, Chassard C, Savary-Auzeloux I. Gut microbes and muscle function: can probiotics make our muscles stronger? J Cachexia Sarcopenia Muscle. 2022. This review provides a summary of the impact of probiotics on muscle mass and function in mice and humans.
Fielding RA, Reeves AR, Jasuja R, Liu C, Barrett BB, Lustgarten MS. Muscle strength is increased in mice that are colonized with microbiota from high-functioning older adults. Exp Gerontol. 2019;127:110722.
Buigues C, Fernandez-Garrido J, Pruimboom L, Hoogland AJ, Navarro-Martinez R, Martinez-Martinez M, et al. Effect of a prebiotic formulation on frailty syndrome: a randomized, double-blind clinical trial. Int J Mol Sci. 2016;17(6).
Fu SK, Tseng WC, Tseng KW, Lai CC, Tsai YC, Tai HL, et al. Effect of daily oral Lactobacillus plantarum PS128 on exercise capacity recovery after a half-marathon. Nutrients. 2021;13(11).
Reijnders D, Goossens GH, Hermes GDA, Smidt H, Zoetendal EG, Blaak EE. Short-term microbiota manipulation and forearm substrate metabolism in obese men: a randomized, double-blind, placebo-controlled trial. Obes Facts. 2018;11(4):318–26.
Mitchell CM, Davy BM, Ponder MA, McMillan RP, Hughes MD, Hulver MW, et al. Prebiotic inulin supplementation and peripheral insulin sensitivity in adults at elevated risk for type 2 diabetes: a pilot randomized controlled trial. Nutrients. 2021;13(9).
Backhed F, Manchester JK, Semenkovich CF, Gordon JI. Mechanisms underlying the resistance to diet-induced obesity in germ-free mice. Proc Natl Acad Sci USA. 2007;104(3):979–84.
Rodriguez J, Delzenne NM. Modulation of the gut microbiota-adipose tissue-muscle interactions by prebiotics. J Endocrinol. 2021;249(1):R1–R23.
Canfora EE, Jocken JW, Blaak EE. Short-chain fatty acids in control of body weight and insulin sensitivity. Nat Rev Endocrinol. 2015;11(10):577–91.
Canfora EE, Meex RCR, Venema K, Blaak EE. Gut microbial metabolites in obesity, NAFLD and T2DM. Nat Rev Endocrinol. 2019;15(5):261–73.
Dalile B, Van Oudenhove L, Vervliet B, Verbeke K. The role of short-chain fatty acids in microbiota-gut-brain communication. Nat Rev Gastroenterol Hepatol. 2019;16(8):461–78.
Frampton J, Murphy KG, Frost G, Chambers ES. Short-chain fatty acids as potential regulators of skeletal muscle metabolism and function. Nat Metab. 2020;2(9):840-8. This review provides an extensive summary of the impact of short-chain fatty acids on muscle.
Walsh ME, Bhattacharya A, Sataranatarajan K, Qaisar R, Sloane L, Rahman MM, Kinter M, van Remmen H. The histone deacetylase inhibitor butyrate improves metabolism and reduces muscle atrophy during aging. Aging Cell. 2015;14(6):957–70.
Scheiman J, Luber JM, Chavkin TA, MacDonald T, Tung A, Pham LD, et al. Meta-omics analysis of elite athletes identifies a performance-enhancing microbe that functions via lactate metabolism. Nat Med. 2019;25(7):1104-9. This research article provides a mechanistic demonstration of the crosstalk between one specific microbe, Veillonella atypica, and the muscle upon exercise.
Chen F, Li Q, Chen Y, Wei Y, Liang J, Song Y, Shi L, Wang J, Mao L, Zhang B, Zhang Z. Association of the gut microbiota and fecal short-chain fatty acids with skeletal muscle mass and strength in children. FASEB J. 2022;36(1):e22109.
Lv WQ, Lin X, Shen H, Liu HM, Qiu X, Li BY, Shen WD, Ge CL, Lv FY, Shen J, Xiao HM, Deng HW. Human gut microbiome impacts skeletal muscle mass via gut microbial synthesis of the short-chain fatty acid butyrate among healthy menopausal women. J Cachexia Sarcopenia Muscle. 2021;12(6):1860–70.
Thibaut MM, Bindels LB. Crosstalk between bile acid-activated receptors and microbiome in entero-hepatic inflammation. Trends Mol Med. 2022;28(3):223-36. This review describes how gut microbes modulate bile acid metabolism and pathways.
Perino A, Demagny H, Velazquez-Villegas L, Schoonjans K. Molecular physiology of bile acid signaling in health, disease, and aging. Physiol Rev. 2021;101(2):683-731. Comprehensive review on bile acids, their metabolism, and their role in health, disease, and aging.
Watanabe M, Houten SM, Mataki C, Christoffolete MA, Kim BW, Sato H, Messaddeq N, Harney JW, Ezaki O, Kodama T, Schoonjans K, Bianco AC, Auwerx J. Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature. 2006;439(7075):484–9.
Sasaki T, Kuboyama A, Mita M, Murata S, Shimizu M, Inoue J, Mori K, Sato R. The exercise-inducible bile acid receptor Tgr5 improves skeletal muscle function in mice. J Biol Chem. 2018;293(26):10322–32.
Abrigo J, Gonzalez F, Aguirre F, Tacchi F, Gonzalez A, Meza MP, et al. Cholic acid and deoxycholic acid induce skeletal muscle atrophy through a mechanism dependent on TGR5 receptor. J Cell Physiol. 2020.
Sasaki T, Watanabe Y, Kuboyama A, Oikawa A, Shimizu M, Yamauchi Y, Sato R. Muscle-specific TGR5 overexpression improves glucose clearance in glucose-intolerant mice. J Biol Chem. 2021;296:100131.
Benoit B, Meugnier E, Castelli M, Chanon S, Vieille-Marchiset A, Durand C, Bendridi N, Pesenti S, Monternier PA, Durieux AC, Freyssenet D, Rieusset J, Lefai E, Vidal H, Ruzzin J. Fibroblast growth factor 19 regulates skeletal muscle mass and ameliorates muscle wasting in mice. Nat Med. 2017;23(8):990–6.
Qiu Y, Yu J, Ji X, Yu H, Xue M, Zhang F, Li Y, Bao Z. Ileal FXR-FGF15/19 signaling activation improves skeletal muscle loss in aged mice. Mech Ageing Dev. 2022;202:111630.
Guo A, Li K, Xiao Q. Fibroblast growth factor 19 alleviates palmitic acid-induced mitochondrial dysfunction and oxidative stress via the AMPK/PGC-1alpha pathway in skeletal muscle. Biochem Biophys Res Commun. 2020;526(4):1069–76.
Koppe L, Pillon NJ, Vella RE, Croze ML, Pelletier CC, Chambert S, Massy Z, Glorieux G, Vanholder R, Dugenet Y, Soula HA, Fouque D, Soulage CO. p-Cresyl sulfate promotes insulin resistance associated with CKD. J Am Soc Nephrol. 2013;24(1):88–99.
Savira F, Cao L, Wang I, Yang W, Huang K, Hua Y, Jucker BM, Willette RN, Huang L, Krum H, Li Z, Fu Q, Wang BH. Apoptosis signal-regulating kinase 1 inhibition attenuates cardiac hypertrophy and cardiorenal fibrosis induced by uremic toxins: implications for cardiorenal syndrome. PLoS ONE. 2017;12(11):e0187459.
Han H, Zhu J, Zhu Z, Ni J, Du R, Dai Y, et al. p-Cresyl sulfate aggravates cardiac dysfunction associated with chronic kidney disease by enhancing apoptosis of cardiomyocytes. J Am Heart Assoc. 2015;4(6):e001852.
Huang TH, Yip HK, Sun CK, Chen YL, Yang CC, Lee FY. P-cresyl sulfate causes mitochondrial hyperfusion in H9C2 cardiomyoblasts. J Cell Mol Med. 2020;24(15):8379–90.
Koh A, Molinaro A, Stahlman M, Khan MT, Schmidt C, Manneras-Holm L, et al. Microbially produced imidazole propionate impairs insulin signaling through mTORC1. Cell. 2018;175(4):947–61 e17.
Roager HM, Licht TR. Microbial tryptophan catabolites in health and disease. Nat Commun. 2018;9(1):3294.
Sato E, Mori T, Mishima E, Suzuki A, Sugawara S, Kurasawa N, Saigusa D, Miura D, Morikawa-Ichinose T, Saito R, Oba-Yabana I, Oe Y, Kisu K, Naganuma E, Koizumi K, Mokudai T, Niwano Y, Kudo T, Suzuki C, et al. Metabolic alterations by indoxyl sulfate in skeletal muscle induce uremic sarcopenia in chronic kidney disease. Sci Rep. 2016;6:36618.
Enoki Y, Watanabe H, Arake R, Sugimoto R, Imafuku T, Tominaga Y, Ishima Y, Kotani S, Nakajima M, Tanaka M, Matsushita K, Fukagawa M, Otagiri M, Maruyama T. Indoxyl sulfate potentiates skeletal muscle atrophy by inducing the oxidative stress-mediated expression of myostatin and atrogin-1. Sci Rep. 2016;6:32084.
Thome T, Salyers ZR, Kumar RA, Hahn D, Berru FN, Ferreira LF, Scali ST, Ryan TE. Uremic metabolites impair skeletal muscle mitochondrial energetics through disruption of the electron transport system and matrix dehydrogenase activity. Am J Physiol Cell Physiol. 2019;317(4):C701–C13.
Lin YL, Liu CH, Lai YH, Wang CH, Kuo CH, Liou HH, Hsu BG. Association of serum indoxyl sulfate levels with skeletal muscle mass and strength in chronic hemodialysis patients: a 2-year longitudinal analysis. Calcif Tissue Int. 2020;107(3):257–65.
Enoki Y, Watanabe H, Arake R, Fujimura R, Ishiodori K, Imafuku T, Nishida K, Sugimoto R, Nagao S, Miyamura S, Ishima Y, Tanaka M, Matsushita K, Komaba H, Fukagawa M, Otagiri M, Maruyama T. Potential therapeutic interventions for chronic kidney disease-associated sarcopenia via indoxyl sulfate-induced mitochondrial dysfunction. J Cachexia Sarcopenia Muscle. 2017;8(5):735–47.
Du L, Qi R, Wang J, Liu Z, Wu Z. Indole-3-propionic acid, a functional metabolite of Clostridium sporogenes, promotes muscle tissue development and reduces muscle cell inflammation. Int J Mol Sci. 2021;22(22).
Agus A, Clement K, Sokol H. Gut microbiota-derived metabolites as central regulators in metabolic disorders. Gut. 2021;70(6):1174–1182. https://pubmed.ncbi.nlm.nih.gov/33272977/
Agudelo LZ, Ferreira DMS, Dadvar S, Cervenka I, Ketscher L, Izadi M, et al. Skeletal muscle PGC-1alpha1 reroutes kynurenine metabolism to increase energy efficiency and fatigue-resistance. Nat Commun. 2019;10(1):2767. This research article demonstrates that upon exercise, PGC-1α1 is activated and coordinates a program that leads to the production of glutamate and an improvement in muscle bioenergetics.
Haikonen R, Karkkainen O, Koistinen V, Hanhineva K. Diet- and microbiota-related metabolite, 5-aminovaleric acid betaine (5-AVAB), in health and disease. Trends Endocrinol Metab. 2022;33:463–80.
Zhao M, Wei H, Li C, Zhan R, Liu C, Gao J, et al. Gut microbiota production of trimethyl-5-aminovaleric acid reduces fatty acid oxidation and accelerates cardiac hypertrophy. Nat Commun. 2022;13(1):1757. Demonstration of the impact of the 5-AVAB on cardiac muscle cells and investigation of the underlying mechanisms.
Karkkainen O, Tuomainen T, Koistinen V, Tuomainen M, Leppanen J, Laitinen T, et al. Whole grain intake associated molecule 5-aminovaleric acid betaine decreases beta-oxidation of fatty acids in mouse cardiomyocytes. Sci Rep. 2018;8(1):13036.
González-Sarrías A, Espín JC, Tomás-Barberán FA. Non-extractable polyphenols produce gut microbiota metabolites that persist in circulation and show anti-inflammatory and free radical-scavenging effects. Trends Food Sci Technol. 2017;69:281–8.
Houghton MJ, Kerimi A, Mouly V, Tumova S, Williamson G. Gut microbiome catabolites as novel modulators of muscle cell glucose metabolism. FASEB J. 2019;33(2):1887-98. In this research article, the authors demonstrated the ability of microbiota-derived phenolic metabolites to modulate glucose uptake and metabolism in human muscle cells.
Rodriguez J, Pierre N, Naslain D, Bontemps F, Ferreira D, Priem F, Deldicque L, Francaux M. Urolithin B, a newly identified regulator of skeletal muscle mass. J Cachexia Sarcopenia Muscle. 2017;8(4):583–97.
Andreux PA, Blanco-Bose W, Ryu D, Burdet F, Ibberson M, Aebischer P, et al. The mitophagy activator urolithin A is safe and induces a molecular signature of improved mitochondrial and cellular health in humans. Nat Metab. 2019;1(6):595-603. This phase I study demonstrates the successful translation of the benefits of the natural food metabolite urolithin A to humans, with an improvement in mitochondrial health of elderly subjects.
Rodriguez J, Caille O, Ferreira D, Francaux M. Pomegranate extract prevents skeletal muscle of mice against wasting induced by acute TNF-alpha injection. Mol Nutr Food Res. 2017;61(4).
Pallister T, Jackson MA, Martin TC, Zierer J, Jennings A, Mohney RP, MacGregor A, Steves CJ, Cassidy A, Spector TD, Menni C. Hippurate as a metabolomic marker of gut microbiome diversity: modulation by diet and relationship to metabolic syndrome. Sci Rep. 2017;7(1):13670.
Huang M, Wei R, Wang Y, Su T, Li P, Chen X. The uremic toxin hippurate promotes endothelial dysfunction via the activation of Drp1-mediated mitochondrial fission. Redox Biol. 2018;16:303–13.
Bitner BF, Ray JD, Kener KB, Herring JA, Tueller JA, Johnson DK, Tellez Freitas CM, Fausnacht DW, Allen ME, Thomson AH, Weber KS, McMillan RP, Hulver MW, Brown DA, Tessem JS, Neilson AP. Common gut microbial metabolites of dietary flavonoids exert potent protective activities in beta-cells and skeletal muscle cells. J Nutr Biochem. 2018;62:95–107.
Bindels LB, Beck R, Schakman O, Martin JC, De Backer FC, Sohet FM, et al. Restoring specific lactobacilli levels decreases inflammation and muscle atrophy markers in an acute leukemia mouse model. PLoS ONE. 2012;7(6):e37971.
Varian BJ, Goureshetti S, Poutahidis T, Lakritz JR, Levkovich T, Kwok C, Teliousis K, Ibrahim YM, Mirabal S, Erdman SE. Beneficial bacteria inhibit cachexia. Oncotarget. 2016;7(11):11803–16.
Song J, Wang C, Long D, Li Z, You L, Brand-Saberi B, Wang G, Yang X. Dysbacteriosis-induced LPS elevation disturbs the development of muscle progenitor cells by interfering with retinoic acid signaling. FASEB J. 2020;34(5):6837–53.
Doyle A, Zhang G, bdel Fattah EA, Eissa NT, Li YP. Toll-like receptor 4 mediates lipopolysaccharide-induced muscle catabolism via coordinate activation of ubiquitin-proteasome and autophagy-lysosome pathways. FASEB J. 2011;25(1):99-110.
Bindels LB, Neyrinck AM, Claus SP, Le Roy CI, Grangette C, Pot B, et al. Synbiotic approach restores intestinal homeostasis and prolongs survival in leukaemic mice with cachexia. ISME J. 2016;10(6):1456–70.
Bindels LB, Neyrinck AM, Loumaye A, Catry E, Walgrave H, Cherbuy C, Leclercq S, van Hul M, Plovier H, Pachikian B, Bermúdez-Humarán LG, Langella P, Cani PD, Thissen JP, Delzenne NM. Increased gut permeability in cancer cachexia: mechanisms and clinical relevance. Oncotarget. 2018;9(26):18224–38.
De Spiegeleer A, Elewaut D, Van Den Noortgate N, Janssens Y, Debunne N, Van Langenhove S, et al. Quorum sensing molecules as a novel microbial factor impacting muscle cells. Biochim Biophys Acta Mol Basis Dis. 2020;1866(3):165646. This research article reported for the first time that some quorum sensing molecules can affect muscle cell viability and inflammation in vitro.
Schwarzer M, Makki K, Storelli G, Machuca-Gayet I, Srutkova D, Hermanova P, Martino ME, Balmand S, Hudcovic T, Heddi A, Rieusset J, Kozakova H, Vidal H, Leulier F. Lactobacillus plantarum strain maintains growth of infant mice during chronic undernutrition. Science. 2016;351(6275):854–7.
Martin A, Ecklu-Mensah G, Ha CWY, Hendrick G, Layman DK, Gilbert J, Devkota S. Gut microbiota mediate the FGF21 adaptive stress response to chronic dietary protein-restriction in mice. Nat Commun. 2021;12(1):3838.
Oost LJ, Kustermann M, Armani A, Blaauw B, Romanello V. Fibroblast growth factor 21 controls mitophagy and muscle mass. J Cachexia Sarcopenia Muscle. 2019;10(3):630–42.
Mardinoglu A, Shoaie S, Bergentall M, Ghaffari P, Zhang C, Larsson E, Bäckhed F, Nielsen J. The gut microbiota modulates host amino acid and glutathione metabolism in mice. Mol Syst Biol. 2015;11(10):834.
Neis EP, Dejong CH, Rensen SS. The role of microbial amino acid metabolism in host metabolism. Nutrients. 2015;7(4):2930–46.
Newsome SD, Feeser KL, Bradley CJ, Wolf C, Takacs-Vesbach C, Fogel ML. Isotopic and genetic methods reveal the role of the gut microbiome in mammalian host essential amino acid metabolism. Proc Biol Sci. 2020;287(1922):20192995.
Ren G, Zhang J, Li M, Tang Z, Yang Z, Cheng G, Wang J. Gut microbiota composition influences outcomes of skeletal muscle nutritional intervention via blended protein supplementation in posttransplant patients with hematological malignancies. Clin Nutr. 2021;40(1):94–102.
Baracos VE, Martin L, Korc M, Guttridge DC, Fearon KCH. Cancer-associated cachexia. Nat Rev Dis Primers. 2018;4:17105.
Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, Jatoi A, Loprinzi C, MacDonald N, Mantovani G, Davis M, Muscaritoli M, Ottery F, Radbruch L, Ravasco P, Walsh D, Wilcock A, Kaasa S, Baracos VE. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol. 2011;12(5):489–95.
Argiles JM, Stemmler B, Lopez-Soriano FJ, Busquets S. Inter-tissue communication in cancer cachexia. Nat Rev Endocrinol. 2018;15(1):9–20.
Schmidt SF, Rohm M, Herzig S, Berriel DM. Cancer cachexia: more than skeletal muscle wasting. Trends Cancer. 2018;4(12):849–60.
Bindels LB, Neyrinck AM, Salazar N, Taminiau B, Druart C, Muccioli GG, François E, Blecker C, Richel A, Daube G, Mahillon J, de los Reyes-Gavilán CG, Cani PD, Delzenne NM. Non digestible oligosaccharides modulate the gut microbiota to control the development of leukemia and associated cachexia in mice. PLoS ONE. 2015;10(6):e0131009.
Potgens SA, Brossel H, Sboarina M, Catry E, Cani PD, Neyrinck AM, et al. Klebsiella oxytoca expands in cancer cachexia and acts as a gut pathobiont contributing to intestinal dysfunction. Sci Rep. 2018;8(1):12321.
Pekkala S, Keskitalo A, Kettunen E, Lensu S, Nykanen N, Kuopio T, et al. Blocking activin receptor ligands is not sufficient to rescue cancer-associated gut microbiota-a role for gut microbial flagellin in colorectal cancer and cachexia? Cancers (Basel). 2019;11(11).
Castellani C, Singer G, Kaiser M, Kaiser T, Huang J, Sperl D, Kashofer K, Fauler G, Guertl-Lackner B, Höfler G, Till H. Neuroblastoma causes alterations of the intestinal microbiome, gut hormones, inflammatory cytokines, and bile acid composition. Pediatr Blood Cancer. 2017;64:e26425.
Jabes DL, de Maria Y, Aciole Barbosa D, Santos K, Carvalho LM, Humberto AC, et al. Fungal dysbiosis correlates with the development of tumor-induced cachexia in mice. J Fungi (Basel). 2020;6(4).
Potgens SA, Thibaut MM, Joudiou N, Sboarina M, Neyrinck AM, Cani PD, et al. Multi-compartment metabolomics and metagenomics reveal major hepatic and intestinal disturbances in cancer cachectic mice. J Cachexia Sarcopenia Muscle. 2021;12(2):456–475. https://pubmed.ncbi.nlm.nih.gov/33599103/
Thibaut MM, Sboarina M, Roumain M, Potgens SA, Neyrinck AM, Destree F, et al. Inflammation-induced cholestasis in cancer cachexia. J Cachexia Sarcopenia Muscle. 2021;12(1):70–90. https://pubmed.ncbi.nlm.nih.gov/33350058. First paper demonstrating an alteration in bile acid pathways in mice and humans with cancer cachexia, and how this altered bile acid profile contributes to hepatic inflammation.
Thibaut MM, Gillard J, Dolly A, Roumain M, Leclercq IA, Delzenne NM, et al. Bile acid dysregulation is intrinsically related to cachexia in tumor-bearing mice. Cancers (Basel). 2021;13(24).
Ni Y, Lohinai Z, Heshiki Y, Dome B, Moldvay J, Dulka E, et al. Distinct composition and metabolic functions of human gut microbiota are associated with cachexia in lung cancer patients. ISME J. 2021. First paper reporting an alteration in the gut microbiota between lung cancer patients classified as well-nourished and patients classified as malnourished (19/12 patients).
Ubachs J, Ziemons J, Soons Z, Aarnoutse R, van Dijk DPJ, Penders J, et al. Gut microbiota and short-chain fatty acid alterations in cachectic cancer patients. J Cachexia Sarcopenia Muscle. 2021. First paper showing a reduction in fecal acetate levels, alongside alterations in the gut microbiota, in patients demonstrating a weight loss of 5% or more within the last 6 months versus weigh stable patients (33/74 patients, diverse cancer types).
Bindels LB, Porporato P, Dewulf EM, Verrax J, Neyrinck AM, Martin JC, Scott KP, Buc Calderon P, Feron O, Muccioli GG, Sonveaux P, Cani PD, Delzenne NM. Gut microbiota-derived propionate reduces cancer cell proliferation in the liver. Br J Cancer. 2012;107(8):1337–44.
Acknowledgements
LBB is the recipient of subsidies from the FSR (Action de Recherche Concertée LIPOCAN, 19-24.096), the Télévie (7.4511.21), from the Walloon Region in the context of the funding of the strategic axis FRFS-WELBIO (40009849), as well as the Fonds Wetenschappelijk Onderzoek – Vlaanderen (FWO) and the Fonds de la Recherche Scientifique – FNRS under EOS Project No. 40007505. CL is a postdoctoral fellow from the Télévie (7.4511.21).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that the review manuscript was written in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Muscle and Bone
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lefevre, C., Bindels, L.B. Role of the Gut Microbiome in Skeletal Muscle Physiology and Pathophysiology. Curr Osteoporos Rep 20, 422–432 (2022). https://doi.org/10.1007/s11914-022-00752-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11914-022-00752-9