Abstract
Purpose of Review
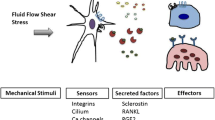
Mechanical loading is an essential stimulus for skeletal tissues. Osteocytes are primarily responsible for sensing mechanical stimuli in bone and for orchestrating subsequent responses. This is critical for maintaining homeostasis, and responding to injury/disease. The osteocyte mechanotransduction pathway, and the downstream effects it mediates, is highly complex. In vivo models have proved invaluable in understanding this process. This review summarizes the commonly used models, as well as more recently developed ones, and describes how they are used to address emerging questions in the field.
Recent Findings
Minimally invasive animal models can be used to determine mechanisms of osteocyte mechanotransduction, at the cell and molecular level, while simultaneously reducing potentially confounding responses such as inflammation/wound-healing.
Summary
The details of osteocyte mechanotransduction in bone are gradually becoming clearer. In vivo model systems are a key tool in pursing this question. Advances in this field are explored and discussed in this review.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Ehrlich PJ, Lanyon LE. Mechanical strain and bone cell function: a review. Osteoporos Int. 2002;13:688–700.
• Schaffler MB, Cheung WY, Majeska R, Kennedy O. Osteocytes: master orchestrators of bone. Calcif Tissue Int. 2014;94:5–24 Provides a detailed review of experimental and theoretical approaches used in osteocyte mechanobiology.
Dallas SL, Bonewald LF. Dynamics of the transition from osteoblast to osteocyte. Ann N Y Acad Sci. 2010;1192:437–43.
Lerner UH. Osteoblasts, osteoclasts, and osteocytes: unveiling their intimate-associated responses to applied orthodontic forces. Remodeling and Modeling of Bone Tissue YSODO. 2012;18:237–48.
Franz-Odendaal TA, Hall BK, Witten PE. Buried alive: how osteoblasts become osteocytes. Dev Dyn. 2006;235:176–90.
Schaffler MB, Kennedy OD. Osteocyte signaling in bone. Curr Osteoporos Rep. 2012;10:118–25.
• Bonewald LF. The amazing osteocyte. J Bone Miner Res. 2011;26:229–38 Provides an excellent review of osteocyte biology and function.
Budyn E, et al. How the morphology of osteocytes contributes to their mechanotransduction near microdamage. MRS Proc. 2015;1724:mrsf14–1724–h12–24.
Kato Y, Windle JJ, Koop BA, Mundy GR, Bonewald LF. Establishment of an osteocyte-like cell line, MLO-Y4. J Bone Miner Res. 2010;12:2014–23.
• Bonewald LF. Establishment and characterization of an osteocyte-like cell line, MLO-Y4. J Bone Miner Metab. 1999;17:61–5 Describes the establishment of the first osteocyte cell line.
Goodship AE, Lanyon LE, McFie H. Functional adaptation of bone to increased stress. An experimental study. J Bone Jt Surgery. 1979;61:539–46.
Lee TC, Staines A, Taylor D. Bone adaptation to load: microdamage as a stimulus for bone remodelling. J Anat. 2002;201:437–46.
Temiyasathit S, Jacobs CR. Osteocyte primary cilium and its role in bone mechanotransduction. Ann N Y Acad Sci. 2010;1192:422–8.
Ducy P, Desbois C, Boyce B, Pinero G, Story B, Dunstan C, et al. Increased bone formation in osteocalcin-deficient mice. Nature. 1996;382:448–52. https://doi.org/10.1038/382448a0.
Wei J, Karsenty G. An overview of the metabolic functions of osteocalcin. Current Osteoporosis Reports. 2015;13:180–5.
Sedillot, C. Des moyens d’assurer la réussite des amputations des membres Résultats statistiques des amputations pratiquées par moi pendant la dernière année scolaire. C R Acad Sci. 1852;34
Hert J, Lisková M, Landa J. Reaction of bone to mechanical stimuli. 1. Continuous and intermittent loading of tibia in rabbit. Praha. 1971;19:290–300.
Hert J, Sklenská A, Lisková M. Reaction of bone to mechanical stimuli. 5. Effect of intermittent stress on the rabbit tibia after resection of the peripheral nerves. Praha. 1971;19:378–87.
Meakin LB, Price JS, Lanyon LE. The contribution of experimental in vivo models to understanding the mechanisms of adaptation to mechanical loading in bone. Front Endocrinol. 2014;5.
Lanyon LE, Rubin CT. Static vs dynamic loads as an influence on bone remodelling. J Biomech. 1984;17:897–905.
• Lanyon LE. Analysis of surface bone strain in the calcaneus of sheep during normal locomotion Strain analysis of the calcaneus. J Biomech. 1973;6:41–9 Describes one of the earliest methods of measuring surface strain of bone in vivo.
Stokes IA, Mente PL, Iatridis JC, Farnum CE, Aronsson DD. Enlargement of growth plate chondrocytes modulated by sustained mechanical loading. J Bone Jt Surg - Ser A. 2002;84:1842–8.
Lambers FM, Koch K, Kuhn G, Ruffoni D, Weigt C, Schulte FA, et al. Trabecular bone adapts to long-term cyclic loading by increasing stiffness and normalization of dynamic morphometric rates. Bone. 2013;55:325–34.
Seref-Ferlengez Z, Basta-Pljakic J, Kennedy OD, Philemon CJ, Schaffler MB. Structural and mechanical repair of diffuse damage in cortical bone in vivo. J Bone Miner Res. 2014;29:2537–44.
Skerry TM, Lanyon LE, Bitensky L, Chayen J. Early strain-related changes in enzyme activity in osteocytes following bone loading in vivo. J Bone Miner Res. 1989;4:783–8.
• Turner CH, Akhter MP, Raab DM, Kimmel DB, Recker RR. A noninvasive, in vivo model for studying strain adaptive bone modeling. Bone. 1991;12:73–9 Describes of the early noninvasive bone adaptation models.
Akhter MP, Cullen DM, Pedersen EA, Kimmel DB, Recker RR. Bone response to in vivo mechanical loading in two breeds of mice. Calcif Tissue Int. 1998;63:442–9.
Gross TS, Srinivasan S, Liu CC, Clemens TL, Bain SD. Noninvasive loading of the murine tibia: an in vivo model for the study of mechanotransduction. J Bone Miner Res. 2002;17:493–501.
Connelly JT, Fritton JC & Van Der Meulen MC Simulation of in vivo loading in the tibia of the C57BL/6 mouse. 49th Annu Meet Orthop Res Soc (2003).
Christiansen BA, Anderson MJ, Lee CA, Williams JC, Yik JHN, Haudenschild DR. Musculoskeletal changes following non-invasive knee injury using a novel mouse model of post-traumatic osteoarthritis. Osteoarthr Cartil. 2012;20:773–82.
Torrance AG, Mosley JR, Suswillo RFL, Lanyon LE. Noninvasive loading of the rat ulna in vivo induces a strain-related modeling response uncomplicated by trauma or periostal pressure. Calcif Tissue Int. 1994;54:241–7.
Lee KCL, Maxwell A, Lanyon LE. Validation of a technique for studying functional adaptation of the mouse ulna in response to mechanical loading. Bone. 2002;31:407–12.
Robling AG, Duijvelaar KM, Geevers JV, Ohashi N, Turner CH. Modulation of appositional and longitudinal bone growth in the rat ulna by applied static and dynamic force. Bone. 2001;29:105–13.
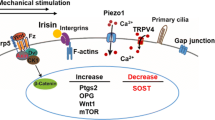
Robling AG, Niziolek PJ, Baldridge LA, Condon KW, Allen MR, Alam I, et al. Mechanical stimulation of bone in vivo reduces osteocyte expression of Sost/sclerostin. J Biol Chem. 2008;283:5866–75.
Lara-Castillo N, et al. In vivo mechanical loading rapidly activates β–catenin signaling in osteocytes through a prostaglandin mediated mechanism HHS public access. Bone. 2015;76:58–66.
Guo D, Bonewald LF. Advancing our understanding of osteocyte cell biology. Therapeutic Advances in Musculoskeletal Disease. 2009;1:87–96.
Spatz JM, Ellman R, Cloutier AM, Louis L, van Vliet M, Suva LJ, et al. Sclerostin antibody inhibits skeletal deterioration due to reduced mechanical loading. J Bone Miner Res. 2013;28:865–74.
Kennedy OD, Laudier DM, Majeska RJ, Sun HB, Schaffler MB. Osteocyte apoptosis is required for production of osteoclastogenic signals following bone fatigue in vivo. Bone. 2014;64:132–7.
Vashishth D. Hierarchy of bone microdamage at multiple length scales. Int J Fatigue. 2007;29:1024–33.
Bellido T, Saini V, Pajevic PD. Effects of PTH on osteocyte function. Bone. 2013;54:250–7.
Verborgt O, Gibson GJ, Schaffler MB. Loss of osteocyte integrity in association with microdamage and bone remodeling after fatigue in vivo. J Bone Miner Res. 2000;15:60–7.
Emerton KB, Hu B, Woo AA, Sinofsky A, Hernandez C, Majeska RJ, et al. Osteocyte apoptosis and control of bone resorption following ovariectomy in mice. Bone. 2010;46:577–83.
Cardoso L, et al. Osteocyte apoptosis controls activation of intracortical resorption in response to bone fatigue. J BONE Miner Res J Bone Min Res. 2009;2424:597–605.
• Fritton JC, Myers ER, Wright TM, Van Der Meulen MCH. Loading induces site-specific increases in mineral content assessed by microcomputed tomography of the mouse tibia. Bone. 2005;36:1030–8 Describes the first tibial loading model to study osteocyte mechanotransduction in trabecular bone.
Morse A, McDonald MM, Kelly NH, Melville KM, Schindeler A, Kramer I, et al. Mechanical load increases in bone formation via a Sclerostin-independent pathway. J Bone Miner Res. 2014;29:2456–67. https://doi.org/10.1002/jbmr.2278.
De Souza RL, et al. Non-invasive axial loading of mouse tibiae increases cortical bone formation and modifies trabecular organization: a new model to study cortical and cancellous compartments in a single loaded element. Bone. 2005;37:810–8.
Brodt MD, Silva MJ. Aged mice have enhanced endocortical response and normal periosteal response compared with young-adult mice following 1 week of axial tibial compression. J Bone Miner Res. 2010;25:2006–15.
Lynch ME, Main RP, Xu Q, Walsh DJ, Schaffler MB, Wright TM, et al. Cancellous bone adaptation to tibial compression is not sex dependent in growing mice. J Appl Physiol. 2010;109:685–91.
Zaman G, Jessop HL, Muzylak M, de Souza RL, Pitsillides AA, Price JS, et al. Osteocytes use estrogen receptor α to respond to strain but their ERα content is regulated by estrogen. J Bone Miner Res. 2006;21:1297–306.
Vashishth D, Koontz J, Qiu SJ, Lundin-Cannon D, Yeni YN, Schaffler MB, et al. In vivo diffuse damage in human vertebral trabecular bone. Bone. 2000;26:147–52.
Furman BD, Strand J, Hembree WC, Ward BD, Guilak F, Olson SA. Joint degeneration following closed intraarticular fracture in the mouse knee: a model of posttraumatic arthritis. J Orthop Res. 2007;25:578–92.
Christiansen BA, Guilak F, Lockwood KA, Olson SA, Pitsillides AA, Sandell LJ, et al. Non-invasive mouse models of post-traumatic osteoarthritis. Osteoarthr Cartil. 2015;23:1627–38.
Lockwood KA, Chu BT, Anderson MJ, Haudenschild DR, Christiansen BA. Comparison of loading rate-dependent injury modes in a murine model of post-traumatic osteoarthritis. J Orthop Res. 2014;32:79–88.
Ramme AJ, Lendhey M, Raya JG, Kirsch T, Kennedy OD. A novel rat model for subchondral microdamage in acute knee injury: a potential mechanism in post-traumatic osteoarthritis. Osteoarthr Cartil. 2016;24:1776–85.
Poulet B, Hamilton RW, Shefelbine S, Pitsillides AA. Characterizing a novel and adjustable noninvasive murine joint loading model. Arthritis Rheum. 2011;63:137–47.
Wu P, Holguin N, Silva MJ, Fu M, Liao W, Sandell LJ. Early response of mouse joint tissue to noninvasive knee injury suggests treatment targets. Arthritis Rheum. 2014;66:1256–65.
Yan H, et al. Suppression of NF-κB activity via nanoparticle-based siRNA delivery alters early cartilage responses to injury. Proc Natl Acad Sci. 2017;114:–E3871.
Rai, M. F. et al. Post-traumatic osteoarthritis in mice following mechanical injury to the synovial joint. Sci Rep 2017;7.
Matheny JB, Goff MG, Pownder SL, Koff MF, Hayashi K, Yang X, et al. An in vivo model of a mechanically-induced bone marrow lesion. J Biomech. 2017;64:258–61.
•• Lewis KJ, et al. Osteocyte calcium signals encode strain magnitude and loading frequency in vivo. Proc Natl Acad Sci. 2017;114:11775–80 Describes a new method of noninvasive mechanical loading to study osteocyte response.
Gu X, Spitzer NC. Distinct aspects of neuronal differentiation encoded by frequency of spontaneous Ca2+ transients. Nature. 1995;375:784–7.
Pereira AF, Javaheri B, Pitsillides AA, Shefelbine SJ. Predicting cortical bone adaptation to axial loading in the mouse tibia. J R Soc Interface. 2015;12:20150590.
Frost H. A determinant of bone architecture. The minimum effective strain. Send to Clin Orthop Relat Res. 1983;175:286–92.
Morey ER, Sabelman EE, Turner RT, Baylink DJ. A new rat model simulating some aspects of space flight. Physiologist. 1979;22:S23–4.
Judex S, Donahue LR, Rubin C. Genetic predisposition to low bone mass is paralleled by an enhanced sensitivity to signals anabolic to the skeleton. FASEB J. 2002;16:1280–2.
Cabahug-Zuckerman P, Frikha-Benayed D, Majeska RJ, Tuthill A, Yakar S, Judex S, et al. Osteocyte apoptosis caused by hind limb unloading is required to trigger osteocyte RANKL production and subsequent resorption of cortical and trabecular bone in mice femurs. J Bone Miner Res. 2016;31:1356–65.
Genetos DC, Geist DJ, Liu D, Donahue HJ, Duncan RL. Fluid shear-induced ATP secretion mediates prostaglandin release in MC3T3-E1 osteoblasts. J Bone Miner Res. 2005;20:41–9.
Forwood MR, Kelly WL, Worth NF. Localization of prostaglandin endoperoxide H synthase (PGHS)-1 and PGHS- 2 in bone following mechanical loading in vivo. Anat Rec. 1998;252:580–6.
Rawlinson SCF, et al. Loading-related increases in prostaglandin production in cores of adult canine cancellous bone in vitro: a role for prostacyclin in adaptive bone remodeling? JBMR. 1991;6:1345–51.
•• Robling, A. G. The interaction of biological factors with mechanical signals in bone adaptation: recent. Reviews recent developments in osteocyte mechanotransduction.
Nguyen AM, Young Y-N, Jacobs CR. The primary cilium is a self-adaptable, integrating nexus for mechanical stimuli and cellular signaling. Biol Open. 2015;4:1733–8.
Hoey DA, Kelly DJ, Jacobs CR. A role for the primary cilium in paracrime signaling between mechanically stimulated osteocytes and mesenchymal stem cells. Biochem Biophys Res Commun Biochem Biophys Res Commun August. 2011;19:182–7.
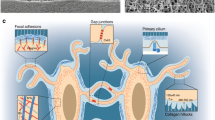
Jacobs CR, Temiyasathit S, Castillo AB. Osteocyte mechanobiology and pericellular mechanics. Annu Rev Biomed Eng. 2010;12:369–400.
Plotkin LI, Speacht TL, Donahue HJ. Cx43 and mechanotransduction in bone. Current Osteoporosis Reports. 2015;13:67–72.
Cabahug-Zuckerman P, Stout RF Jr, Majeska RJ, Thi MM, Spray DC, Weinbaum S, et al. Potential role for a specialized β3 integrin-based structure on osteocyte processes in bone mechanosensation. J Orthop Res. 2018;36:642–52.
Gudi S, Nolan JP, Frangos JA. Modulation of GTPase activity of G proteins by fluid shear stress and phospholipid composition. Proc Natl Acad Sci U S A. 1998;95:2515–9.
Zhang Y-L, Frangos JA, Chachisvilis M. Mechanical stimulus alters conformation of type 1 parathyroid hormone receptor in bone cells. Am J Phys Cell Phys. 2009;296:C1391–9.
Chachisvilis M, Zhang Y-L, Frangos JA. G protein-coupled receptors sense fluid shear stress in endothelial cells. Proc Natl Acad Sci U S A. 2006;103:15463–8.
Javaheri B, Stern AR, Lara N, Dallas M, Zhao H, Liu Y, et al. Deletion of a single β-catenin allele in osteocytes abolishes the bone anabolic response to loading. J Bone Miner Res. 2014;29:705–15.
Kang KS, Robling AG. New insights into Wnt-Lrp5/6-β-catenin signaling in mechanotransduction. Front Endocrinol. 2015;6:246.
•• Bonewald LF. The role of the osteocyte in bone and nonbone disease. Endocrinol Metab Clin N Am. 2017;46:1–18 Describes an updated review of role of osteocytes in multiple pathological states.
Robling AG, Burr DB, Turner CH. Skeletal loading in animals. J Musculoskelet Neuronal Interact. 2001;1:249–62.
Acknowledgements
The authors gratefully acknowledge the contribution of Jack Roberts on the illustrative work in this paper.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Paige V Hinton, Susan M. Rackard, and Oran D. Kennedy declare no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Biomechanics
Rights and permissions
About this article
Cite this article
Hinton, P.V., Rackard, S.M. & Kennedy, O.D. In Vivo Osteocyte Mechanotransduction: Recent Developments and Future Directions. Curr Osteoporos Rep 16, 746–753 (2018). https://doi.org/10.1007/s11914-018-0485-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11914-018-0485-1