Abstract
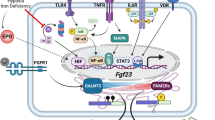
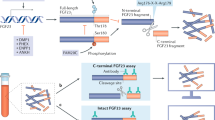
Fibrous dysplasia (FD) is a skeletal disorder caused by activating mutations in Gsα that result in elevations in cAMP. A feature of FD is elevated blood levels of the bone cell-derived phosphaturic hormone, fibroblast growth factor-23 (FGF23). FGF23 regulates serum phosphorus and active vitamin D levels by action on proximal renal tubule cells. An essential step in the production of biologically active FGF23 is glycosylation by the UDP-N-acetyl-α-D-galactosamine:polypeptide N-acetylgalactosaminyl transferase (ppGalNAc-T3). In the absence of glycosylation, FGF23 is processed into inactive N- and C-terminal proteins by a subtilisin proprotein convertase, probably furin. Normally, most if not all circulating FGF23 is intact. In FD, C-terminal levels are elevated, suggesting altered FGF23 processing. Altered processing in FD is the result of a cAMP-dependent, coordinated decrease in ppGalNAc-T3 and an increase in furin enzyme activity. These findings, and emerging data from other diseases, suggest regulation of FGF23 processing may be a physiologically important process.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Lichtenstein L. Poloyostotic fibrous dysplasia. Arch Surg. 1938;36:874–98.
Lichtenstein L, Jaffe HL. Fibrous dysplasia of bone: a condition affecting one, several, or many bones, the graver cases of which may present abnormal pigmentation of skin, premature sexual development, hyperthyroidism, or still other extraskeletal abnormalities. Arch Pathol. 1942;33:777–816.
Collins MT, Bianco P. In: Favus MJ, editor. Primer on the metabolic bone diseases and disorders of mineral metabolism. Chapter 72. American Society for Bone and Mineral Research. Washington, DC; 2006. p.415–8.
McCune D. Osteitis fibrosa cystica; the case of a nine-year-old girl who also exhibits precocious puberty, multiple pigmentation of the skin, and hyperthyroidism. Am J Dis Child. 1960;52:743–4. 1936.
Albright FBA, Hampton AO, Smith P. Syndrome characterized by osteitis fibrosa disseminata, areas of pigmentation, and endocrine dysfunction, with precocious puberty in females: report of 5 cases. N Engl J Med. 1937;216:727–46.
Dumitrescu CE, Collins MT. McCune-Albright syndrome. Orphanet J Rare Dis. 2008;3:12.
Collins MT, Singer FR, Eugster E. McCune-Albright syndrome and the extraskeletal manifestations of fibrous dysplasia. Orphanet J Rare Dis. 2012;7 Suppl 1:S4. doi:10.1186/1750-1172-7-S1-S4.
Weinstein LS et al. Activating mutations of the stimulatory G protein in the McCune-Albright syndrome. N Engl J Med. 1991;325:1688–95.
Schwindinger WF, Francomano CA, Levine MA. Identification of a mutation in the gene encoding the α subunit of the stimulatory G protein of adenylyl cyclase in McCune-Albright syndrome. Proc Natl Acad Sci U S A. 1992;89:5152–6.
Happle R. The McCune-Albright syndrome: a lethal gene surviving by mosaicism. Clin Genet. 1986;29:321–4.
Riminucci M, Saggio I, Robey PG, Bianco P. Fibrous dysplasia as a stem cell disease. J Bone Miner Res. 2006;21 Suppl 2:P125–31.
Weinstein LS. G(s)alpha mutations in fibrous dysplasia and McCune-Albright syndrome. J Bone Miner Res. 2006;21 Suppl 2:P120–4.
Landis CA et al. GTPase inhibiting mutations activate the α chain of Gs and stimulate adenylyl cyclase in human pituitary tumors. Nature. 1989;340:692–6.
Iiri T, Herzmark P, Nakamoto JM, van Dop C, Bourne HR. Rapid GDP release from Gs alpha in patients with gain and loss of endocrine function. Nature. 1994;371:164–8. doi:10.1038/371164a0.
Riminucci M et al. Fibrous dysplasia of bone in the McCune-Albright syndrome: abnormalities in bone formation. Am J Pathol. 1997;151:1587–600.
Riminucci M et al. The histopathology of fibrous dysplasia of bone in patients with activating mutations of the Gs alpha gene: site-specific patterns and recurrent histological hallmarks. J Pathol. 1999;187:249–58.
Corsi A et al. Osteomalacic and hyperparathyroid changes in fibrous dysplasia of bone: core biopsy studies and clinical correlations. J Bone Miner Res. 2003;18:1235–46.
Bianco P et al. Mutations of the GNAS1 gene, stromal cell dysfunction, and osteomalacic changes in non-McCune-Albright fibrous dysplasia of bone. J Bone Miner Res. 2000;15:120–8.
Leet AI et al. Fracture incidence in polyostotic fibrous dysplasia and the McCune-Albright syndrome. J Bone Miner Res. 2004;19:571–7.
Hart ES et al. Onset, progression, and plateau of skeletal lesions in fibrous dysplasia and the relationship to functional outcome. J Bone Miner Res. 2007;22:1468–74.
Kelly MH, Brillante B, Collins MT. Pain in fibrous dysplasia of bone: age-related changes and the anatomical distribution of skeletal lesions. Osteoporos Int. 2008;19:57–63.
Collins MT et al. An instrument to measure skeletal burden and predict functional outcome in fibrous dysplasia of bone. J Bone Miner Res. 2005;20:219–26.
Akintoye SO et al. Characterization of gsp-mediated growth hormone excess in the context of McCune-Albright syndrome. J Clin Endocrinol Metab. 2002;87:5104–12.
Boyce AM et al. Optic neuropathy in McCune-Albright Syndrome: effects of early diagnosis and treatment of growth hormone excess. J Clin Endocrinol Metab. 2012. doi:10.1210/jc.2012–2111.
Prader A, Illig R, Uehlinger E, Stalder G. Rickets following bone tumor. Helv Paediatr Acta. 1959;14:554–65.
•• Chong WH, Molinolo AA, Chen CC, Collins MT. Tumor-induced osteomalacia. Endocr Relat Cancer. 2011;18:R53–77. This article is a thorough review of tumor-induced osteomalacia (TIO) that includes guidance on the diagnosis, tumor localization, and when necessary, medical treatment of TIO.
de Beur SM J. Tumor-induced osteomalacia. JAMA. 2005;294:1260–7.
Autosomal Dominant Hypophosphatemic Rickets (ADHR) Consortium. Autosomal dominant hypophosphataemic rickets is associated with mutations in FGF23. The ADHR Consortium. Nat Genet. 2000;26:345–8.
White KE et al. The autosomal dominant hypophosphatemic rickets (ADHR) gene is a secreted polypeptide overexpressed by tumors that cause phosphate wasting. J Clin Endocrinol Metab. 2001;86:497–500.
Jonsson KB et al. Fibroblast growth factor 23 in oncogenic osteomalacia and X-linked hypophosphatemia. N Engl J Med. 2003;348:1656–63.
Riminucci M et al. FGF-23 in fibrous dysplasia of bone and its relationship to renal phosphate wasting. J Clin Invest. 2003;112:683–92.
Collins MT et al. Renal phosphate wasting in fibrous dysplasia of bone is part of a generalized renal tubular dysfunction similar to that seen in tumor-induced osteomalacia. J Bone Miner Res. 2001;16:806–13.
Dent CE, Gertner JM. Hypophosphataemic osteomalacia in fibrous dysplasia. Q J Med. 1976;45:411–20.
Mirams M, Robinson BG, Mason RS, Nelson AE. Bone as a source of FGF23: regulation by phosphate? Bone. 2004;35:1192–9.
Sitara D et al. Homozygous ablation of fibroblast growth factor-23 results in hyperphosphatemia and impaired skeletogenesis, and reverses hypophosphatemia in Phex-deficient mice. Matrix Biol. 2004;23:421–32.
Feng JQ et al. Loss of DMP1 causes rickets and osteomalacia and identifies a role for osteocytes in mineral metabolism. Nat Genet. 2006;38:1310–5.
Kuznetsov SA et al. Age-dependent demise of GNAS-mutated skeletal stem cells and "normalization" of fibrous dysplasia of bone. J Bone Miner Res. 2008;23:1731–40.
Shimada T et al. Cloning and characterization of FGF23 as a causative factor of tumor-induced osteomalacia. Proc Natl Acad Sci U S A. 2001;98:6500–5.
Shimada T et al. FGF-23 is a potent regulator of vitamin D metabolism and phosphate homeostasis. J Bone Miner Res. 2004;19:429–35.
Shimada T et al. Targeted ablation of Ffg23 demonstrates an essential physiological role of FGF23 in pohsphate and vitamin D metabolism. J Clin Invest. 2004;113:561–8.
Larsson T, Nisbeth U, Ljunggren O, Juppner H, Jonsson KB. Circulating concentration of FGF-23 increases as renal function declines in patients with chronic kidney disease, but does not change in response to variation in phosphate intake in healthy volunteers. Kidney Int. 2003;64:2272–9.
Ferrari SL, Bonjour JP, Rizzoli R. Fibroblast growth factor-23 relationship to dietary phosphate and renal phosphate handling in healthy young men. J Clin Endocrinol Metab. 2005;90:1519–24.
Nishida Y et al. Acute effect of oral phosphate loading on serum fibroblast growth factor 23 levels in healthy men. Kidney Int. 2006;70:2141–7. doi:10.1038/sj.ki.5002000.
Ito N et al. Effect of acute changes of serum phosphate on fibroblast growth factor (FGF)23 levels in humans. J Bone Miner Metab. 2007;25:419–22.
Collins MT et al. Fibroblast growth factor-23 is regulated by 1alpha,25-dihydroxyvitamin D. J Bone Miner Res. 2005;20:1944–50.
Brownstein CA et al. A translocation causing increased alpha-klotho level results in hypophosphatemic rickets and hyperparathyroidism. Proc Natl Acad Sci U S A. 2008;105:3455–60.
• Smith RC et al. Circulating alphaKlotho influences phosphate handling by controlling FGF23 production. J Clin Invest. 2012;122:4710–5. doi:10.1172/JCI64986. The preceding two articles (Brownsetin et al and Smith et al) provide important evidence for a direct role for circulating α-Klotho in the regulation of FGF23.
Rafaelsen SH et al. Exome sequencing reveals FAM20c mutations associated with FGF23-related hypophosphatemia, dental anomalies and ectopic calcification. J Bone Miner Res. 2013. doi:10.1002/jbmr.1850.
Yamashita T, Yoshioka M, Itoh N. Identification of a novel fibroblast growth factor, FGF-23, preferentially expressed in the ventrolateral thalamic nucleus of the brain. Biochem Biophys Res Commun. 2000;277:494–8.
Urakawa I, et al. Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature. 2006;444(7120):770–4.
Kurosu H et al. Regulation of fibroblast growth factor-23 signaling by klotho. J Biol Chem. 2006;281:6120–3.
•• Faul C et al. FGF23 induces left ventricular hypertrophy. J Clin Invest. 2011;121:4393–408. doi:10.1172/JCI46122. This is an excellent article that provides both clinical and experimental evidence for the “off-target,” ie, non-mineral metabolism effects of FGF23 in the pathophysiology of FGF23-mediated cardiovascular disease in renal failure.
Gupta A, Winer K, Econs MJ, Marx SJ, Collins MT. FGF-23 is elevated by chronic hyperphosphatemia. J Clin Endocrinol Metab. 2004;89:4489–92.
Weinman EJ, Steplock D, Shenolikar S, Biswas R. Fibroblast growth factor-23-mediated inhibition of renal phosphate transport in mice requires sodium-hydrogen exchanger regulatory factor-1 (NHERF-1) and synergizes with parathyroid hormone. J Biol Chem. 2011;286:37216–21. doi:10.1074/jbc.M111.288357.
Weinman EJ, Lederer ED. NHERF-1 and the regulation of renal phosphate reabsoption: a tale of three hormones. Am J Physiol. 2012;303:F321–7. doi:10.1152/ajprenal.00093.2012.
Kato K et al. Polypeptide GalNAc-transferase T3 and familial tumoral calcinosis. Secretion of fibroblast growth factor 23 requires O-glycosylation. J Biol Chem. 2006;281:18370–7.
•• Bhattacharyya N, Chong WH, Gafni RI, Collins MT. Fibroblast growth factor 23: state of the field and future directions. Trends Endocrinol Metab. 2012;23:610–8. doi:10.1016/j.tem.2012.07.002. This article provides an excellent overview of the subject of FGF23, highlighting key papers in the development of our current understanding of the physiology and pathophysiology of FGF23 and pointing to the future direction to which the current research is leading.
Gram Schjoldager KT et al. A systematic study of site-specific GalNAc-type O-glycosylation modulating proprotein convertase processing. J Biol Chem. 2011;286:40122–32. doi:10.1074/jbc.M111.287912.
Fukumoto S. Post-translational modification of Fibroblast Growth Factor 23. Ther Apher Dial. 2005;9:319–22.
Frishberg Y et al. Hyperostosis-hyperphosphatemia syndrome: a congenital disorder of O-glycosylation associated with augmented processing of fibroblast growth factor 23. J Bone Miner Res. 2007;22:235–42.
• Bhattacharyya N et al. Mechanism of FGF23 processing in fibrous dysplasia. J Bone Miner Res. 2012;27:1132–41. doi:10.1002/jbmr.1546. This paper is an important paper that details the mechanism of regulation of FGF23 processing in fibrous dysplasia. It points to the potentially important role FGF23 processing may play in mineral metaoblism physiology.
Yuan B et al. Hexa-D-arginine treatment increases 7B2*PC2 activity in hyp-mouse osteoblasts and rescues the HYP phenotype. J Bone Miner Res. 2013;28:56–72. doi:10.1002/jbmr.1738.
Topaz O et al. Mutations in GALNT3, encoding a protein involved in O-linked glycosylation, cause familial tumoral calcinosis. Nat Genet. 2004;36:579–81.
Benet-Pages A, Orlik P, Strom TM, Lorenz-Depiereux B. An FGF23 missense mutation causes familial tumoral calcinosis with hyperphosphatemia. Hum Mol Genet. 2005;14(3):385–90.
Ichikawa S et al. A homozygous missense mutation in human KLOTHO causes severe tumoral calcinosis. J Clin Invest. 2007;117:2684–91.
Bergwitz C et al. Defective O-glycosylation due to a novel homozygous S129P mutation is associated with lack of fibroblast growth factor 23 secretion and tumoral calcinosis. J Clin Endocrinol Metab. 2009;94:4267–74.
Semenov AG et al. Processing of pro-brain natriuretic peptide is suppressed by O-glycosylation in the region close to the cleavage site. Clin Chem. 2009;55:489–98.
Brown WW et al. Hypophosphatemia with elevations in serum fibroblast growth factor 23 in a child with Jansen's metaphyseal chondrodysplasia. J Clin Endocrinol Metab. 2009;94:17–20.
Durham BH, Joseph F, Bailey LM, Fraser WD. The association of circulating ferritin with serum concentrations of fibroblast growth factor-23 measured by three commercial assays. Ann Clin Biochem. 2007;44:463–6.
Imel EA et al. Iron modifies plasma FGF23 differently in autosomal dominant hypophosphatemic rickets and healthy humans. J Clin Endocrinol Metab. 2011;96:3541–9. doi:10.1210/jc.2011-1239.
Farrow EG et al. Iron deficiency drives an autosomal dominant hypophosphatemic rickets (ADHR) phenotype in fibroblast growth factor-23 (Fgf23) knock-in mice. Proc Natl Acad Sci U S A. 2011;108:E1146–55. doi:10.1073/pnas.1110905108.
Goetz R, et al. Isolated C-terminal tail of FGF23 alleviates hypophosphatemia by inhibiting FGF23-FGFR-Klotho complex formation. Proc Natl Acad Sci U S A. 2010;107(1):407–12.
Ichikawa S, Austin AM, Gray AK, Econs MJ. A Phex mutation in a murine model of X-linked hypophosphatemia alters phosphate responsiveness of bone cells. J Bone Miner Res. 2012;27:453–60. doi:10.1002/jbmr.544.
Acknowledgment
This manuscript was supported by Division of Intramural Research of the National Institute of Dental and Craniofacial Research, National Institutes of Health, Department of Health and Human services (A.M.B., N.B., and M.T.C.), and the Bone Health Program at Children’s National Medical Center (A.M.B.).
Disclosure
A.M. Boyce declares no conflicts of interest. N. Bhattacharyya declares no conflicts of interest. M.T. Collins declares no conflicts of interest.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Boyce, A.M., Bhattacharyya, N. & Collins, M.T. Fibrous Dysplasia and Fibroblast Growth Factor-23 Regulation. Curr Osteoporos Rep 11, 65–71 (2013). https://doi.org/10.1007/s11914-013-0144-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11914-013-0144-5