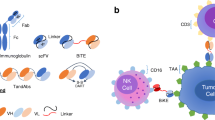
Abstract
Purpose of Review
The purpose of this review is to discuss the current recommendations for the use of bispecific antibodies (bsAb) in hematologic malignancies and explore the future in this field.
Recent Findings
Bispecific antibodies are molecules able to target two different antigen-binding sites: one towards a tumor antigen and another to activate a cytotoxic cell. Phase II/III trials on blinatumomab for acute lymphoblastic leukemia (ALL) have demonstrated its efficacy for treating minimal residual disease (MRD+) and relapsed refractory (r/r) Philadelphia positive (Ph+) and negative (Ph−) ALL in adults and children.
Summary
Currently, the only bispecific antibody (bsAb) approved for its use in hematologic malignancies is blinatumomab. However, multiple trials are under development not only to explore blinatumomab’s clinical activity in other neoplasia, such as lymphoma or multiple myeloma, but also to develop new molecules against different antigens.
Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Yang Y. Cancer immunotherapy harnessing the immune system to battle cancer. J Clin Invest. 2015;125(9):3335–7.
Redman JM, Hill EM, AlDeghaither D, Weiner LM. Mechanism of action of therapeutic antibodies for cancer. Mol Immunol. 2015;67(2):28–45.
Staerz UD, Kanagawa O, Bevan MJ. Hybrid antibodies can target sites for attack by T cells. Nature. 1985;314:628–31.
de Gast GC, Haagen IA, van Houten AA, Klein SC, Duits AJ, de Weger RA, et al. CD8 T cell activation after intravenous administration of CD3 × CD19 bispecific antibodies in patients with non-Hodgkin lymphoma. Cancer Immunol Immunother. 1995;40:390–6.
• Brinkmann U, Kontermann RE. The making of bispecific antibodies. MAbs. 2017;9:182–212 Interesting review regarding the history, structure, and activity of bsAb.
Krishnarmurthy A, Jimenez A. Bispecific antibodies for cancer therapy: a review. Pharmacol Ther. 2017;185:122–34.
Viardot A, Bargou R. Bispecific antibodies in haematological malignancies. Cancer Treat Rev. 2018;65:87–95.
Nagorsen D, Kufer P, Baeuerle PA, Bargou R. Blinatumomab: a historical perspective. Pharmacol Ther. 2012;136(3):334–42.
Goebler ME, Bargou R. Blinatumomab: a CD19/CD3 bispecific T cell engager (BiTE) with unique anti-tumor efficacy. Leuk Lymphoma. 2016;57(5):1021–32.
Goebler ME, Knop S, Viardot A, Kufer P, Topp MS, Einsele h NR, et al. Bispecific T-cell engager (BiTE) antibody construct blinatumomab for the treatment of patients with relapsed/refractory non Hodgkin lymphoma: final results from a phase I study. J Clin Oncol. 2016;34(10):1104–11.
Topp MS, Gökbuget N, Zugmaier G, Degenhard E, Goebler ME, Klinger M, et al. Long-term follow-up of hematologic relapse-free survival in a phase 2 study of blinatumomab in patients with MRD in B-lineage ALL. Blood. 2012;120(26):5185–7.
Gökbuget N, Dombret H, Bonifacio M, Reichle A, Graux C, Faul C, et al. Blinatumomab for minimal residual disease in adults with B-cell precursor acute lymphoblastic leukemia. Blood. 2018;131(14):1522–31.
Handgretinger R, Zugmaier G, Henze G, Kreyenberg H, Lang P, von Stackelberg A. Complete remission after blinatumomab-induced donor T-cell activation in three pediatric patients with post-transplant relapsed acute lymphoblastic leukemia. Leukemia. 2011;25(1):181–4.
Topp MS, GÖkbuget N, Zugmaier G, Klappers P, Stelljes M, Neumann S, et al. Phase II trial of anti-C19 bispecific T cell-engager blinatumomab shows hematologic and molecular remissions in patients with relapsed or refractory B-precursor acute lymphobastic leukemia. J Clin Oncol. 2014;32(36):4134–40.
Topp MS, Gökbuget N, Stein AS, Zugmaier G, O’Brien S, Bargou R, et al. Safety and activity of blinatumomab for adult patients with relapsed or refractory B-precursor acute lymphoblastic leukemia: a multicenter, single-arm, phase 2 study. Lancet Oncol. 2015;16:57–66.
• Kantarjian H, Stein A, Gökbuget N, Fielding AK, Schuh AC, Ribera JM, et al. Blinatumomab versus chemotherapy for advanced acute lymphoblastic leukemia. N Engl J Med. 2017;376:836–47 Phase III clinical trial that compared Blinatumomab to standard chemotherapy. Demonstrated that Blinatumomab group achieved higher rates of CR, OS, and RFS.
• Von Stackelberg A, Locatelli F, Zugmaier G, Handgretinger R, Trippett TM, Rizzari C, et al. Phase I/phase II study of blinatumomab in pediatric patients with relapsed/refractory acute lymphoblastic leukemia. J Clin Oncol. 2016;34(36):4381–9 Phase I/II clinical trial able to demonstrate the activity of Blinatumomab in the r/r pediatric setting.
Martinelli G, Boissel N, Chevallier P, Ottmann O, Gökbuget N, Topp MS, et al. Complete hematologic and molecular response in adult patients with relapsed/refractory Philadelphia chromosome-positive B-precursor acute lymphoblastic leukemia following treatment with Blinatumomab: results from a phase II, single arm, multicenter study. J Clin Oncol. 2017;35(16):1795–802.
Assi R, Kantarjian H, Short NJ, Daver N, Takahashi K, Garcia-Manero G, et al. Safety and efficacy of blinatumomab in combination with a tyrosine kinase inhibitor for the treatment of relapsed Philadelphia chromosome-positive leukemia. Clin Lymphoma Myeloma Leuk. 2017;17(12):897–901.
Blincyto (blinatumomab) [prescribing information]. Thousand Oaks, CA: Amgen Inc; 2014. https://www.blincyto.com.
Viardot A, Goebeler ME, Hess G, Neumann S, Pfreundschuh M, Adrian N, et al. Phase 2 study of the bispecific T-cell engager (BiTE) antibody blinatumomab in relapsed/refractory diffuse large B-cell lymphoma. Blood. 2016;127(11):1410–6.
Löffler A, Gruen M, Wuchter C, Schriever F, Kufer P, Dreier T, et al. Efficient elimination of chronic lymphocytic leukaemia B cells by autologous T cells with a bispecific anti-CD19/anti-CD3 single-chain antibody construct. Leukemia. 2003;17(5):900–9.
Wong R, Pepper C, Brennan P, Nagorsen D, Man S, Fegan C. Blinatumomab induces autologous T-cell killing of chronic lymphocytic leukemia cells. Haematologica. 2013;98(12):1930–8.
Reusch U, Duell J, Ellwanger K, Herbrecht C, Knackmuss S, Fucek I, et al. A tetravalent bispecific TandAb (CD19/CD3), AFM11, efficiently recruits T cells for the potent lysis of CD19+ tumor cells. MAbs. 2015;7:584–604.
Liu L, Lam CK, Long V, Widjaja L, Yang Y, Li H. MGD011, a CD19 × CD3 dual affinity retargeting bi-specific molecule incorporating extended circulating half-life for the treatment of B-cell malignancies. Clin Cancer Res. 2017;23:1506–18.
Buhmann R, Michael S, Juergen H, Horst L, Peschel C, Kolb HJ. Immunotherapy with FBTA05 (Bi20), a trifunctional bispecific anti-CD3 × anti-CD20 antibody and donor lymphocyte infusion (DLI) in relapsed or refractory B-cell lymphoma after allogeneic stem cell transplantation: study protocol of an investigator-driven, open-label, non-randomized, uncontrolled, dose-escalating Phase I/II-trial. J Transl Med. 2013;11:160.
Schuster FR, Stanglmaier M, Woessmann W, Winkler B, Siepermann M, Meisel R, et al. Immunotherapy with the trifunctional anti-CD20 × anti-CD3 antibody FBTA05 (Lymphomun) in paediatric high-risk patients with recurrent CD20-positive B cell malignancies. Br J Haematol. 2015;169:90–102.
Kieslich A, Ruf P, Lindhofer H, Buhmann R, Eggert A, Hundsdoerfer P. Immunotherapy with the trifunctional anti-CD20 × anti-CD3 antibody FBTA05 in a patient with relapsed t(8;14)-positive post-transplant lymphoproliferative disease. Leuk Lymphoma. 2017;58:1989–92.
Bannerji R, Advani RH, Brown JR, Arnason JE, Barnes JA, Allan JN, et al. Safety and preliminary clinical activity of REGN1979, an anti-CD20 x anti-CD3 bispecific antibody, in patients with B-NHL previously treated with CD20-directed antibody therapy. Blood. 2017;130:1550.
Topp MS, Borchmann P, Wagner-Johnston ND, Provencio M, Cordoba R. Papadopoulos K. Safety and Preliminary antitumor activity of the anti-PD-1 monoclonal antibody REGN2810 alone or in combination with REGN1979, an anti-CD20 x anti-CD3 bispecific antibody, in patients with B-lymphoid malignancies. Blood. 2017;130:1495.
Bacac M, Umaña P, Herter S, Colombetti S, Sam J, Le Clech M, et al. CD20 Tcb (RG6026), a novel “2:1” T cell bispecific antibody for the treatment of B cell malignancies. Blood. 2016;129:1836.
Rothe A, Sasse S, Topp MS, Eichenauer DA, Hummel H, Reiners KS, et al. A phase 1 study of the bispecific anti-CD30/CD16A antibody construct AFM13 in patients with relapsed or refractory Hodgkin lymphoma. Blood. 2015;125:4024–31.
Hipp S, Tai YT, Blanset D, Deegen P, Wahl J, Thomas O, et al. A novel BCMA/CD3 bispecific T-cell engager for the treatment of multiple myeloma induces selective lysis in vitro and in vivo. Leukemia. 2017;31:1743–51.
Seckinger A, Delgado JA, Moser S, Moreno L, Neuber B, Grab A, et al. Target expression, generation, preclinical activity, and pharmacokinetics of the BCMA-T cell bispecific antibody EM801 for multiple myeloma treatment. Cancer Cell. 2017;31:396–410.
De Zafra C, Balazs M, Fajardo F, Liang L, Zhong W, Henn A, et al. Preclinical characterization of AMG 424, a novel humanized T cell-recruiting bispecific anti-CD3/CD38 antibody. Blood. 2017;129:500.
Chu SY, Miranda Y, Phung S, Chen H, Rashid R, Endo NA, et al. Immunotherapy with long-lived anti-CD38 × anti-CD3 bispecific antibodies stimulates potent T cell-mediated killing of human myeloma cell lines and CD38+ cells in monkeys: a potential therapy for multiple myeloma. Blood. 2014;124:4727.
Moore GL, Lee SH, Schubbert S, Miranda Y, Rashid R, Pong E, et al. Tuning T cell affinity improves efficacy and safety of anti-CD38 × anti-CD3 bispecific antibodies in monkeys—a potential therapy for multiple myeloma. Blood. 2015;126:1798.
Hoseini SS, Cheung NK. Acute myeloid leukemia targets for bispecific antibodies. Blood. Cancer J. 2017;7:e522. https://doi.org/10.1038/bcj.2017.35.
Krupka C, Kufer P, Kischel R, Zugmaier G, Bogeholz J, Kohnke T, et al. CD33 target validation and sustained depletion of AML blasts in long-term cultures by the bispecific T-cell-engaging antibody AMG 330. Blood. 2014;123:356–65.
Aigner M, Feulner J, Schaffer S, Kischel R, Kufer P, Schneider K, et al. T lymphocytes can be effectively recruited for ex vivo and in vivo lysis of AML blasts by a novel CD33/CD3-bispecific BiTE antibody construct. Leukemia. 2013;27:1107–15.
Laszlo GS, Gudgeon CJ, Harrington KH, Dell’Aringa J, Newhall KJ, Means GD, et al. Cellular determinants for preclinical activity of a novel CD33/CD3 bispecific T-cell engager (BiTE) antibody, AMG 330, against human AML. Blood. 2014;123:554–61.
Uy GL, Godwin J, Rettig MP, Vey N, Foster M, Arellano ML, et al. Preliminary results of a phase 1 study of flotetuzumab, a CD123 x CD3 bispecific Dart® protein, in patients with relapsed/refractory acute myeloid leukemia and myelodysplastic syndrome. Blood. 2017;130(Suppl 1):637.
Rettig MP, Godwin J, Vey N, Fox B, Ballesteros-Merino C, Bifulco CB, et al. Preliminary translational results from an ongoing phase 1 study of flotetuzumab, a CD123 x CD3 Dart®, in AML/MDS: rationale for combining flotetuzumab and anti-PD-1/PD-L1 immunotherapies. Blood. 2017;130(Suppl 1):1365.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Roberta Demichelis-Gómez has received research funding through grants from Amgen and Novartis; has received speakers’ honoraria from AbbVie, Amgen, Celgene, Novartis, and Shire; has received reimbursement for travel expenses from AbbVie and Amgen; and has received compensation from AbbVie and Novartis for participation on advisory boards.
Daniela Pérez-Sámano declares that she has no conflict of interest.
Christianne Bourlon has received speakers’ honoraria from Shire.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Leukemia
Rights and permissions
About this article
Cite this article
Demichelis-Gómez, R., Pérez-Sámano, D. & Bourlon, C. Bispecific Antibodies in Hematologic Malignancies: When, to Whom, and How Should Be Best Used?. Curr Oncol Rep 21, 17 (2019). https://doi.org/10.1007/s11912-019-0759-5
Published:
DOI: https://doi.org/10.1007/s11912-019-0759-5