Abstract
Purpose of Review
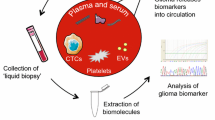
Given the invasive and high-risk nature of brain surgery, the need for non-invasive biomarkers obtained from the peripheral blood is greatest in tumors of the central nervous system (CNS). In this comprehensive review, we highlight recent advances in blood biomarker development for adult and pediatric brain tumors.
Recent Findings
We summarize recent blood biomarker development for CNS tumors across multiple key analytes, including peripheral blood mononuclear cells, cell-free DNA, cell-free RNA, proteomics, circulating tumor cells, and tumor-educated platelets. We also discuss methods for enhancing blood biomarker detection through transient opening of the blood-brain barrier.
Summary
Although blood-based biomarkers are not yet used in routine neuro-oncology practice, this field is advancing rapidly and holds great promise for improved and non-invasive management of patients with brain tumors. Prospective and adequately powered studies are needed to confirm the clinical utility of any blood biomarker prior to widespread clinical implementation.
Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Grossman SA, Ye X, Lesser G, Sloan A, Carraway H, Desideri S, et al. Immunosuppression in patients with high-grade gliomas treated with radiation and temozolomide. Clin Cancer Res. 2011;17(16):5473–80. https://doi.org/10.1158/1078-0432.Ccr-11-0774.
Valero C, Lee M, Hoen D, Weiss K, Kelly DW, Adusumilli PS, et al. Pretreatment neutrophil-to-lymphocyte ratio and mutational burden as biomarkers of tumor response to immune checkpoint inhibitors. Nat Commun. 2021;12(1):729. https://doi.org/10.1038/s41467-021-20935-9.
Bagley SJ, Kothari S, Aggarwal C, Bauml JM, Alley EW, Evans TL, et al. Pretreatment neutrophil-to-lymphocyte ratio as a marker of outcomes in nivolumab-treated patients with advanced non-small-cell lung cancer. Lung Cancer. 2017;106:1–7. https://doi.org/10.1016/j.lungcan.2017.01.013.
Howard R, Kanetsky PA, Egan KM. Exploring the prognostic value of the neutrophil-to-lymphocyte ratio in cancer. Sci Rep. 2019;9(1):19673. https://doi.org/10.1038/s41598-019-56218-z.
Clavreul A, Lemée JM, Soulard G, Rousseau A, Menei P. A simple preoperative blood count to stratify prognosis in isocitrate dehydrogenase-wildtype glioblastoma patients treated with radiotherapy plus concomitant and adjuvant temozolomide. Cancers (Basel). 2021;13(22). https://doi.org/10.3390/cancers13225778.
Marini A, Dobran M, Aiudi D, Pesaresi A, di Somma LGM, Iacoangeli M. Pre-operative hematological markers as predictive factors for overall survival and progression free survival in glioblastomas. Clin Neurol Neurosurg. 2020;197:106162. https://doi.org/10.1016/j.clineuro.2020.106162.
Stoyanov GS, Lyutfi E, Georgieva R, Dzhenkov DL, Petkova L, Ivanov BD, et al. The role of preoperative neutrophil, platelet, and monocyte to lymphocyte ratios as independent prognostic factors for patient survival in WHO 2021 glioblastoma: a single-center retrospective study. Cureus. 2022;14(6):e25801. https://doi.org/10.7759/cureus.25801.
Cote DJ, Creed JH, Samanic CM, Gerke TA, Stampfer MJ, Smith-Warner SA, et al. A prospective study of inflammatory biomarkers and growth factors and risk of glioma in the UK Biobank. Cancer Epidemiol. 2021;75:102043. https://doi.org/10.1016/j.canep.2021.102043.
Guo X, Jiao H, Zhang T, Zhang Y. Pre-treatment and preoperative neutrophil-to-lymphocyte ratio predicts prognostic value of glioblastoma: a meta-analysis. Brain Sci. 2022;12(5). https://doi.org/10.3390/brainsci12050675.
Gomes Dos Santos A, de Carvalho RF, de Morais A, Silva TM, Baylão VMR, Azevedo M, et al. Role of neutrophil-lymphocyte ratio as a predictive factor of glioma tumor grade: a systematic review. Crit Rev Oncol Hematol. 2021;163:103372. https://doi.org/10.1016/j.critrevonc.2021.103372.
Yang C, Wen HB, Zhao YH, Huang WH, Wang ZF, Li ZQ. Systemic inflammatory indicators as prognosticators in glioblastoma patients: a comprehensive meta-analysis. Front Neurol. 2020;11:580101. https://doi.org/10.3389/fneur.2020.580101.
Bonilla DL, Reinin G, Chua E. Full spectrum flow cytometry as a powerful technology for cancer immunotherapy research. Front Mol Biosci. 2020;7:612801. https://doi.org/10.3389/fmolb.2020.612801.
van der Pan K, Khatri I, de Jager AL, Louis A, Kassem S, Naber BAE, et al. Performance of spectral flow cytometry and mass cytometry for the study of innate myeloid cell populations. Front Immunol. 2023;14:1191992. https://doi.org/10.3389/fimmu.2023.1191992.
Park LM, Lannigan J, Jaimes MC. OMIP-069: Forty-color full spectrum flow cytometry panel for deep immunophenotyping of major cell subsets in human peripheral blood. Cytometry A. 2020;97(10):1044–51. https://doi.org/10.1002/cyto.a.24213.
Jaimes MC, Leipold M, Kraker G, Amir EA, Maecker H, Lannigan J. Full spectrum flow cytometry and mass cytometry: a 32-marker panel comparison. Cytometry A. 2022;101(11):942–59. https://doi.org/10.1002/cyto.a.24565.
Majzner RG, Ramakrishna S, Yeom KW, Patel S, Chinnasamy H, Schultz LM, et al. GD2-CAR T cell therapy for H3K27M-mutated diffuse midline gliomas. Nature. 2022;603(7903):934–41. https://doi.org/10.1038/s41586-022-04489-4.
Das A, Tabori U, Sambira Nahum LC, Collins NB, Deyell R, Dvir R, et al. Efficacy of nivolumab in pediatric cancers with high mutation burden and mismatch-repair deficiency. Clin Cancer Res. 2023. https://doi.org/10.1158/1078-0432.Ccr-23-0411.
Cloughesy TF, Mochizuki AY, Orpilla JR, Hugo W, Lee AH, Davidson TB, et al. Neoadjuvant anti-PD-1 immunotherapy promotes a survival benefit with intratumoral and systemic immune responses in recurrent glioblastoma. Nat Med. 2019;25(3):477–86. https://doi.org/10.1038/s41591-018-0337-7.
Ghosh S, Huang J, Inkman M, Zhang J, Thotala S, Tikhonova E, et al. Radiation-induced circulating myeloid-derived suppressor cells induce systemic lymphopenia after chemoradiotherapy in patients with glioblastoma. Sci Transl Med. 2023;15(680):eabn6758. https://doi.org/10.1126/scitranslmed.abn6758.
Del Bianco P, Pinton L, Magri S, Canè S, Masetto E, Basso D, et al. Myeloid diagnostic and prognostic markers of immune suppression in the blood of glioma patients. Front Immunol. 2021;12:809826. https://doi.org/10.3389/fimmu.2021.809826.
Salas LA, Zhang Z, Koestler DC, Butler RA, Hansen HM, Molinaro AM, et al. Enhanced cell deconvolution of peripheral blood using DNA methylation for high-resolution immune profiling. Nat Commun. 2022;13(1):761. https://doi.org/10.1038/s41467-021-27864-7.
Molinaro AM, Wiencke JK, Warrier G, Koestler DC, Chunduru P, Lee JY, et al. Interactions of age and blood immune factors and noninvasive prediction of glioma survival. J Natl Cancer Inst. 2022;114(3):446–57. https://doi.org/10.1093/jnci/djab195.
Wiencke JK, Molinaro AM, Warrier G, Rice T, Clarke J, Taylor JW, et al. DNA methylation as a pharmacodynamic marker of glucocorticoid response and glioma survival. Nat Commun. 2022;13(1):5505. https://doi.org/10.1038/s41467-022-33215-x.
Diehl F, Li M, Dressman D, He Y, Shen D, Szabo S, et al. Detection and quantification of mutations in the plasma of patients with colorectal tumors. Proc Natl Acad Sci U S A. 2005;102(45):16368–73. https://doi.org/10.1073/pnas.0507904102.
Gobbini E, Swalduz A, Giaj Levra M, Ortiz-Cuaran S, Toffart A-C, Pérol M, et al. Implementing ctDNA analysis in the clinic: challenges and opportunities in non-small cell lung cancer. Cancers. 2020;12(11):3112.
Leighl NB, Page RD, Raymond VM, Daniel DB, Divers SG, Reckamp KL, et al. Clinical utility of comprehensive cell-free DNA analysis to identify genomic biomarkers in patients with newly diagnosed metastatic non-small cell lung cancer. Clin Cancer Res. 2019;25(15):4691–700. https://doi.org/10.1158/1078-0432.Ccr-19-0624.
Bagley SJ, Nabavizadeh SA, Mays JJ, Till JE, Ware JB, Levy S, et al. Clinical utility of plasma cell-free DNA in adult patients with newly diagnosed glioblastoma: a pilot prospective study. Clin Cancer Res. 2020;26(2):397–407. https://doi.org/10.1158/1078-0432.Ccr-19-2533.
Bagley SJ, Till J, Abdalla A, Sangha HK, Yee SS, Freedman J, et al. Association of plasma cell-free DNA with survival in patients with IDH wild-type glioblastoma. Neuro-Oncology. Advances. 2021;3(1). https://doi.org/10.1093/noajnl/vdab011.
Fontanilles M, Marguet F, Beaussire L, Magne N, Pépin LF, Alexandru C, et al. Cell-free DNA and circulating TERT promoter mutation for disease monitoring in newly-diagnosed glioblastoma. Acta Neuropathol Commun. 2020;8(1):179. https://doi.org/10.1186/s40478-020-01057-7.
Izquierdo E, Proszek P, Pericoli G, Temelso S, Clarke M, Carvalho DM, et al. Droplet digital PCR-based detection of circulating tumor DNA from pediatric high grade and diffuse midline glioma patients. Neurooncol Adv. 2021;3(1):vdab013. https://doi.org/10.1093/noajnl/vdab013.
Mouliere F, Smith CG, Heider K, Su J, van der Pol Y, Thompson M, et al. Fragmentation patterns and personalized sequencing of cell-free DNA in urine and plasma of glioma patients. EMBO Mol Med. 2021;13(8):e12881. https://doi.org/10.15252/emmm.202012881.
Bonner ER, Harrington R, Eze A, Bornhorst M, Kline CN, Gordish-Dressman H, et al. Circulating tumor DNA sequencing provides comprehensive mutation profiling for pediatric central nervous system tumors. npj Precision. Oncology. 2022;6(1):63. https://doi.org/10.1038/s41698-022-00306-3.
Pagès M, Rotem D, Gydush G, Reed S, Rhoades J, Ha G, et al. Liquid biopsy detection of genomic alterations in pediatric brain tumors from cell-free DNA in peripheral blood, CSF, and urine. Neuro-Oncology. 2022;24(8):1352–63. https://doi.org/10.1093/neuonc/noab299. This study represents the largest cohort of pediatric brain tumors to be assessed by liquid biopsy NGS.
Mutter JA, Alig SK, Esfahani MS, Lauer EM, Mitschke J, Kurtz DM, et al. Circulating tumor DNA profiling for detection, risk stratification, and classification of brain lymphomas. J Clin Oncol. 2023;41(9):1684–94. https://doi.org/10.1200/jco.22.00826. The authors report a study of 119 plasma samples in patients with primary CNS lymphoma, demonstrating that ctDNA can be detected reliably in the plasma of patients with this disease and is associated with poor clinical prognosis.
Panditharatna E, Kilburn LB, Aboian MS, Kambhampati M, Gordish-Dressman H, Magge SN, et al. Clinically relevant and minimally invasive tumor surveillance of pediatric diffuse midline gliomas using patient-derived liquid biopsy. Clin Cancer Res. 2018;24(23):5850–9. https://doi.org/10.1158/1078-0432.Ccr-18-1345.
Li D, Bonner ER, Wierzbicki K, Panditharatna E, Huang T, Lulla R, et al. Standardization of the liquid biopsy for pediatric diffuse midline glioma using ddPCR. Sci Rep. 2021;11(1):5098. https://doi.org/10.1038/s41598-021-84513-1.
Cantor E, Wierzbicki K, Tarapore RS, Ravi K, Thomas C, Cartaxo R, et al. Serial H3K27M cell-free tumor DNA (cf-tDNA) tracking predicts ONC201 treatment response and progression in diffuse midline glioma. Neuro Oncol. 2022;24(8):1366–74. https://doi.org/10.1093/neuonc/noac030. This study performed serial plasma sampling and detected H3K27M circulating tumor DNA in 53/62 plasma samples. The authors also observed “spikes” in the ctDNA levels preceding progression in 50% of cases.
Muralidharan K, Yekula A, Small JL, Rosh ZS, Kang KM, Wang L, et al. TERT promoter mutation analysis for blood-based diagnosis and monitoring of gliomas. Clin Cancer Res. 2021;27(1):169–78. https://doi.org/10.1158/1078-0432.Ccr-20-3083.
Kang KM, Muralidharan K, Yekula A, Small JL, Rosh ZS, Jones PS, et al. Blood-based detection of BRAF V600E in gliomas and brain tumor metastasis. Cancers (Basel). 2021;13(6). https://doi.org/10.3390/cancers13061227.
García-Romero N, Carrión-Navarro J, Areal-Hidalgo P, Ortiz de Mendivil A, Asensi-Puig A, Madurga R, et al. BRAF V600E detection in liquid biopsies from pediatric central nervous system tumors. Cancers. 2020;12(1):66.
Christodoulou E, Yellapantula V, O’Halloran K, Xu L, Berry JL, Cotter JA, et al. Combined low-pass whole genome and targeted sequencing in liquid biopsies for pediatric solid tumors. NPJ Precis Oncol. 2023;7(1):21. https://doi.org/10.1038/s41698-023-00357-0.
Liu APY, Smith KS, Kumar R, Paul L, Bihannic L, Lin T, et al. Serial assessment of measurable residual disease in medulloblastoma liquid biopsies. Cancer Cell. 2021;39(11):1519–30.e4. https://doi.org/10.1016/j.ccell.2021.09.012.
Capper D, Jones DTW, Sill M, Hovestadt V, Schrimpf D, Sturm D, et al. DNA methylation-based classification of central nervous system tumours. Nature. 2018;555(7697):469–74. https://doi.org/10.1038/nature26000.
Nassiri F, Chakravarthy A, Feng S, Shen SY, Nejad R, Zuccato JA, et al. Detection and discrimination of intracranial tumors using plasma cell-free DNA methylomes. Nat Med. 2020;26(7):1044–7. https://doi.org/10.1038/s41591-020-0932-2.
Sabedot TS, Malta TM, Snyder J, Nelson K, Wells M, deCarvalho AC, et al. A serum-based DNA methylation assay provides accurate detection of glioma. Neuro-Oncology. 2021;23(9):1494–508. https://doi.org/10.1093/neuonc/noab023.
Maire CL, Fuh MM, Kaulich K, Fita KD, Stevic I, Heiland DH, et al. Genome-wide methylation profiling of glioblastoma cell-derived extracellular vesicle DNA allows tumor classification. Neuro-Oncology. 2021;23(7):1087–99. https://doi.org/10.1093/neuonc/noab012.
Herrgott GA, Asmaro KP, Wells M, Sabedot TS, Malta TM, Mosella MS, et al. Detection of tumor-specific DNA methylation markers in the blood of patients with pituitary neuroendocrine tumors. Neuro-Oncology. 2022;24(7):1126–39. https://doi.org/10.1093/neuonc/noac050.
Larson MH, Pan W, Kim HJ, Mauntz RE, Stuart SM, Pimentel M, et al. A comprehensive characterization of the cell-free transcriptome reveals tissue- and subtype-specific biomarkers for cancer detection. Nat Commun. 2021;12(1):2357. https://doi.org/10.1038/s41467-021-22444-1.
El-Hefnawy T, Raja S, Kelly L, Bigbee WL, Kirkwood JM, Luketich JD, et al. Characterization of amplifiable, circulating RNA in plasma and its potential as a tool for cancer diagnostics. Clin Chem. 2004;50(3):564–73. https://doi.org/10.1373/clinchem.2003.028506.
Lo KW, Lo YM, Leung SF, Tsang YS, Chan LY, Johnson PJ, et al. Analysis of cell-free Epstein-Barr virus associated RNA in the plasma of patients with nasopharyngeal carcinoma. Clin Chem. 1999;45(8 Pt 1):1292–4.
Kopreski MS, Benko FA, Kwak LW, Gocke CD. Detection of tumor messenger RNA in the serum of patients with malignant melanoma. Clin Cancer Res. 1999;5(8):1961–5.
Skog J, Würdinger T, van Rijn S, Meijer DH, Gainche L, Curry WT, et al. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol. 2008;10(12):1470–6. https://doi.org/10.1038/ncb1800.
Ohno M, Matsuzaki J, Kawauchi J, Aoki Y, Miura J, Takizawa S, et al. Assessment of the diagnostic utility of serum microRNA classification in patients with diffuse glioma. JAMA Netw Open. 2019;2(12):e1916953-e. https://doi.org/10.1001/jamanetworkopen.2019.16953.
Morokoff A, Jones J, Nguyen H, Ma C, Lasocki A, Gaillard F, et al. Serum microRNA is a biomarker for post-operative monitoring in glioma. J Neuro-Oncol. 2020;149(3):391–400. https://doi.org/10.1007/s11060-020-03566-w.
Bustos MA, Rahimzadeh N, Ryu S, Gross R, Tran LT, Renteria-Lopez VM, et al. Cell-free plasma microRNAs that identify patients with glioblastoma. Lab Investig. 2022;102(7):711–21. https://doi.org/10.1038/s41374-021-00720-4.
Swellam M, Ezz El Arab L, Al-Posttany AS, S BS. Clinical impact of circulating oncogenic MiRNA-221 and MiRNA-222 in glioblastoma multiform. J Neuro-Oncol. 2019;144(3):545–51. https://doi.org/10.1007/s11060-019-03256-2.
Lan F, Yue X, Xia T. Exosomal microRNA-210 is a potentially non-invasive biomarker for the diagnosis and prognosis of glioma. Oncol Lett. 2020;19(3):1967–74. https://doi.org/10.3892/ol.2020.11249.
Díaz Méndez AB, Sacconi A, Tremante E, Lulli V, Caprara V, Rosanò L, et al. A diagnostic circulating miRNA signature as orchestrator of cell invasion via TKS4/TKS5/EFHD2 modulation in human gliomas. J Exp Clin Cancer Res. 2023;42(1):66. https://doi.org/10.1186/s13046-023-02639-8.
Liu ZZ, Tian YF, Wu H, Ouyang SY, Kuang WL. LncRNA H19 promotes glioma angiogenesis through miR-138/HIF-1α/VEGF axis. Neoplasma. 2020;67(1):111–8. https://doi.org/10.4149/neo_2019_190121N61.
Jia P, Cai H, Liu X, Chen J, Ma J, Wang P, et al. Long non-coding RNA H19 regulates glioma angiogenesis and the biological behavior of glioma-associated endothelial cells by inhibiting microRNA-29a. Cancer Lett. 2016;381(2):359–69. https://doi.org/10.1016/j.canlet.2016.08.009.
Tan SK, Pastori C, Penas C, Komotar RJ, Ivan ME, Wahlestedt C, et al. Serum long noncoding RNA HOTAIR as a novel diagnostic and prognostic biomarker in glioblastoma multiforme. Mol Cancer. 2018;17(1):74. https://doi.org/10.1186/s12943-018-0822-0.
Amer RG, Ezz El Arab LR, Abd El Ghany D, Saad AS, Bahie-Eldin N, Swellam M. Prognostic utility of lncRNAs (LINC00565 and LINC00641) as molecular markers in glioblastoma multiforme (GBM). J Neuro-Oncol. 2022;158(3):435–44. https://doi.org/10.1007/s11060-022-04030-7.
Ita MI, Wang JH, Toulouse A, Lim C, Fanning N, O'Sullivan M, et al. The utility of plasma circulating cell-free messenger RNA as a biomarker of glioma: a pilot study. Acta Neurochir. 2022;164(3):723–35. https://doi.org/10.1007/s00701-021-05014-8.
Puigdelloses M, González-Huárriz M, García-Moure M, Martínez-Vélez N, Esparragosa Vázquez I, Bruna J, et al. RNU6-1 in circulating exosomes differentiates GBM from non-neoplastic brain lesions and PCNSL but not from brain metastases. Neurooncol Adv. 2020;2(1):vdaa010. https://doi.org/10.1093/noajnl/vdaa010.
Batool SM, Muralidharan K, Hsia T, Falotico S, Gamblin AS, Rosenfeld YB, et al. Highly sensitive EGFRvIII detection in circulating extracellular vesicle RNA of glioma patients. Clin Cancer Res. 2022;28(18):4070–82. https://doi.org/10.1158/1078-0432.Ccr-22-0444.
Aziz N, Nishanian P, Mitsuyasu R, Detels R, Fahey JL. Variables that affect assays for plasma cytokines and soluble activation markers. Clin Diagn Lab Immunol. 1999;6(1):89–95. https://doi.org/10.1128/cdli.6.1.89-95.1999.
Vitanza NA, Johnson AJ, Wilson AL, Brown C, Yokoyama JK, Künkele A, et al. Locoregional infusion of HER2-specific CAR T cells in children and young adults with recurrent or refractory CNS tumors: an interim analysis. Nat Med. 2021;27(9):1544–52. https://doi.org/10.1038/s41591-021-01404-8.
Vitanza NA, Wilson AL, Huang W, Seidel K, Brown C, Gustafson JA, et al. Intraventricular B7-H3 CAR T cells for diffuse intrinsic pontine glioma: preliminary first-in-human bioactivity and safety. Cancer Discov. 2023;13(1):114–31. https://doi.org/10.1158/2159-8290.Cd-22-0750.
Liu S, Zhu Y, Zhang C, Meng X, Sun B, Zhang G, et al. The clinical significance of soluble programmed cell death-ligand 1 (sPD-L1) in patients with gliomas. Front Oncol. 2020;10:9. https://doi.org/10.3389/fonc.2020.00009.
Mair MJ, Ilhan-Mutlu A, Pajenda S, Kiesel B, Wöhrer A, Widhalm G, et al. Circulating PD-L1 levels change during bevacizumab-based treatment in recurrent glioma. Cancer Immunol Immunother. 2021;70(12):3643–50. https://doi.org/10.1007/s00262-021-02951-2.
Jiguet-Jiglaire C, Boissonneau S, Denicolai E, Hein V, Lasseur R, Garcia J, et al. Plasmatic MMP9 released from tumor-infiltrating neutrophils is predictive for bevacizumab efficacy in glioblastoma patients: an AVAglio ancillary study. Acta Neuropathol Commun. 2022;10(1):1. https://doi.org/10.1186/s40478-021-01305-4.
Khristov V, Nesterova D, Trifoi M, Clegg T, Daya A, Barrett T, et al. Plasma IL13Rα2 as a novel liquid biopsy biomarker for glioblastoma. J Neuro-Oncol. 2022;160(3):743–52. https://doi.org/10.1007/s11060-022-04196-0.
Osti D, Del Bene M, Rappa G, Santos M, Matafora V, Richichi C, et al. Clinical significance of extracellular vesicles in plasma from glioblastoma patients. Clin Cancer Res. 2019;25(1):266–76. https://doi.org/10.1158/1078-0432.Ccr-18-1941.
Indira Chandran V, Welinder C, Månsson AS, Offer S, Freyhult E, Pernemalm M, et al. Ultrasensitive immunoprofiling of plasma extracellular vesicles identifies syndecan-1 as a potential tool for minimally invasive diagnosis of glioma. Clin Cancer Res. 2019;25(10):3115–27. https://doi.org/10.1158/1078-0432.Ccr-18-2946.
Brennan PM, Butler HJ, Christie L, Hegarty MG, Jenkinson MD, Keerie C, et al. Early diagnosis of brain tumours using a novel spectroscopic liquid biopsy. Brain Commun. 2021;3(2):fcab056. https://doi.org/10.1093/braincomms/fcab056.
Butler HJ, Brennan PM, Cameron JM, Finlayson D, Hegarty MG, Jenkinson MD, et al. Development of high-throughput ATR-FTIR technology for rapid triage of brain cancer. Nat Commun. 2019;10(1):4501. https://doi.org/10.1038/s41467-019-12527-5.
Müller C, Holtschmidt J, Auer M, Heitzer E, Lamszus K, Schulte A, et al. Hematogenous dissemination of glioblastoma multiforme. Sci Transl Med. 2014;6(247):247ra101. https://doi.org/10.1126/scitranslmed.3009095.
Lynch D, Powter B, Po JW, Cooper A, Garrett C, Koh E-S, et al. Isolation of circulating tumor cells from glioblastoma patients by direct immunomagnetic targeting. Appl Sci. 2020;10(9):3338.
Müller Bark J, Kulasinghe A, Hartel G, Leo P, Warkiani ME, Jeffree RL, et al. Isolation of circulating tumour cells in patients with glioblastoma using spiral microfluidic technology - a pilot study. Front Oncol. 2021;11:681130. https://doi.org/10.3389/fonc.2021.681130.
In ‘t Veld SGJG, Wurdinger T. Tumor-educated platelets. Blood. 2019;133(22):2359–64. https://doi.org/10.1182/blood-2018-12-852830.
In 't Veld S, Arkani M, Post E, Antunes-Ferreira M, D'Ambrosi S, DCL V, et al. Detection and localization of early- and late-stage cancers using platelet RNA. Cancer Cell. 2022;40(9):999–1009.e6. https://doi.org/10.1016/j.ccell.2022.08.006.
Sol N, In 't Veld S, Vancura A, Tjerkstra M, Leurs C, Rustenburg F, et al. Tumor-educated platelet RNA for the detection and (pseudo)progression monitoring of glioblastoma. Cell Rep Med. 2020;1(7):100101. https://doi.org/10.1016/j.xcrm.2020.100101.
Rincon-Torroella J, Khela H, Bettegowda A, Bettegowda C. Biomarkers and focused ultrasound: the future of liquid biopsy for brain tumor patients. J Neuro-Oncol. 2022;156(1):33–48. https://doi.org/10.1007/s11060-021-03837-0.
Meng Y, Pople CB, Suppiah S, Llinas M, Huang Y, Sahgal A, et al. MR-guided focused ultrasound liquid biopsy enriches circulating biomarkers in patients with brain tumors. Neuro-Oncology. 2021;23(10):1789–97. https://doi.org/10.1093/neuonc/noab057. This was a first-in-human study using FUS for enhanced blood-based liquid biopsy.
Yuan J, Xu L, Chien CY, Yang Y, Yue Y, Fadera S, et al. First-in-human prospective trial of sonobiopsy in glioblastoma patients using neuronavigation-guided focused ultrasound. medRxiv. 2023. https://doi.org/10.1101/2023.03.17.23287378.
Funding
The authors did not receive support from any organization for the submitted work, and no funding was received to assist with the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
J.F, M.K, and S.B. wrote the main manuscript text.
Corresponding author
Ethics declarations
Competing Interests
JF has received in-kind research support from Tempus. MK has received personal fees as a scientific advisory board member for HTG Molecular Diagnostics. SB has received in-kind research support from Tempus, C2i Genomics, and Guardant Health.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Foster, J.B., Koptyra, M.P. & Bagley, S.J. Recent Developments in Blood Biomarkers in Neuro-oncology. Curr Neurol Neurosci Rep 23, 857–867 (2023). https://doi.org/10.1007/s11910-023-01321-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11910-023-01321-y