Abstract
Purpose of the Review
Brain metastasis is a common complication of advanced malignancies, especially, lung cancer, breast cancer, renal cell carcinoma, and melanoma. Traditionally surgery, when indicated, and radiation therapy, either as whole-brain radiation therapy or stereotactic radiosurgery, constituted the major treatment options for brain metastases. Until recently, most of the systemic chemotherapy agents had limited activity for brain metastases. However, with the advent of small molecule tyrosine kinase inhibitors and immunotherapy agents, there has been renewed interest in using these agents in the management of brain metastases.
Recent Findings
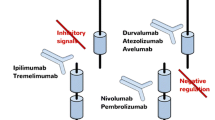
Immune checkpoint inhibitors have revolutionized the treatment of metastatic melanoma, lung cancer, kidney cancer, and bladder cancer among others. They modulate the immune system to recognize tumor antigens as “non-self” antigens and mount an immune response against them.
Summary
Initial studies of using immune checkpoint inhibitors in brain metastases have shown promising activity, and several clinical trials are currently underway. Studies are also assessing the combination of radiation therapy and immunotherapy in brain metastases. The results of these ongoing clinical trials have the potential to change the therapeutic paradigm in patients with brain metastases.
Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Raizer JJ, Hwu W-J, Panageas KS, Wilton A, Baldwin DE, Bailey E, et al. Brain and leptomeningeal metastases from cutaneous melanoma: survival outcomes based on clinical features. Neuro-Oncol. 2008;10:199–207.
Schouten LJ, Rutten J, Huveneers HAM, Twijnstra A. Incidence of brain metastases in a cohort of patients with carcinoma of the breast, colon, kidney, and lung and melanoma. Cancer. 2002;94:2698–705.
Khasraw M, Posner JB. Neurological complications of systemic cancer. Lancet Neurol. 2010;9:1214–27.
Venur VA, Ahluwalia MS. Prognostic scores for brain metastasis patients: use in clinical practice and trial design. Chin Clin Oncol. 2015;4:18.
Sperduto PW, Yang TJ, Beal K, Pan H, Brown PD, Bangdiwala A, et al. Estimating survival in patients with lung cancer and brain metastases: an update of the graded prognostic assessment for lung cancer using molecular markers (lung-molGPA). JAMA Oncol. 2017;3:827–31.
Sperduto PW, Jiang W, Brown PD, Braunstein S, Sneed P, Wattson DA, et al. The prognostic value of BRAF, C-KIT, and NRAS mutations in melanoma patients with brain metastases. Int J Radiat Oncol Biol Phys. 2017;98:1069–77.
Sperduto PW, Kased N, Roberge D, Xu Z, Shanley R, Luo X, et al. Effect of tumor subtype on survival and the graded prognostic assessment for patients with breast cancer and brain metastases. Int J Radiat Oncol Biol Phys. 2012;82:2111–7.
Patchell RA, Tibbs PA, Walsh JW, Dempsey RJ, Maruyama Y, Kryscio RJ, et al. A randomized trial of surgery in the treatment of single metastases to the brain. N Engl J Med. 1990;322:494–500.
Vecht CJ, Haaxma-Reiche H, Noordijk EM, Padberg GW, Voormolen JH, Hoekstra FH, et al. Treatment of single brain metastasis: radiotherapy alone or combined with neurosurgery? Ann Neurol. 1993;33:583–90.
Andrews DW, Scott CB, Sperduto PW, Flanders AE, Gaspar LE, Schell MC, et al. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial. Lancet Lond Engl. 2004;363:1665–72.
Aoyama H, Shirato H, Tago M, Nakagawa K, Toyoda T, Hatano K, et al. Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases: a randomized controlled trial. JAMA. 2006;295:2483–91.
Kocher M, Soffietti R, Abacioglu U, Villà S, Fauchon F, Baumert BG, et al. Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: results of the EORTC 22952-26001 study. J Clin Oncol. 2011;29:134–41.
Chang EL, Wefel JS, Hess KR, Allen PK, Lang FF, Kornguth DG, et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. Lancet Oncol. 2009;10:1037–44.
Tsao MN, Lloyd N, Wong RKS, Chow E, Rakovitch E, Laperriere N, et al. Whole brain radiotherapy for the treatment of newly diagnosed multiple brain metastases. Cochrane Database Syst Rev. 2012;4:CD003869.
Agarwala SS, Kirkwood JM, Gore M, Dreno B, Thatcher N, Czarnetski B, et al. Temozolomide for the treatment of brain metastases associated with metastatic melanoma: a phase II study. J Clin Oncol Off J Am Soc Clin Oncol. 2004;22:2101–7.
Wu Y-L, Zhou C, Cheng Y, Lu S, Chen G-Y, Huang C, et al. Erlotinib as second-line treatment in patients with advanced non-small-cell lung cancer and asymptomatic brain metastases: a phase II study (CTONG–0803). Ann Oncol. 2013;24:993–9.
Venur VA, Ahluwalia MS. Targeted therapy in brain metastases: ready for primetime? Am Soc Clin Oncol Educ Book Am Soc Clin Oncol Meet. 2016;35:e123–30.
Yap YS, Cornelio GH, Devi BCR, Khorprasert C, Kim SB, Kim TY, et al. Brain metastases in Asian HER2-positive breast cancer patients: anti-HER2 treatments and their impact on survival. Br J Cancer. 2012;107:1075–82.
Long GV, Trefzer U, Davies MA, Kefford RF, Ascierto PA, Chapman PB, et al. Dabrafenib in patients with Val600Glu or Val600Lys BRAF-mutant melanoma metastatic to the brain (BREAK-MB): a multicentre, open-label, phase 2 trial. Lancet Oncol. 2012;13:1087–95.
McArthur GA, Maio M, Arance A, Nathan P, Blank C, Avril M-F, et al. Vemurafenib in metastatic melanoma patients with brain metastases: an open-label, single-arm, phase 2, multicentre study. Ann Oncol Off J Eur Soc Med Oncol. 2017;28:634–41.
Davies MA, Robert C, Long GV, Grob JJ, Flaherty KT, Arance A, et al. COMBI-MB: a phase II study of combination dabrafenib (D) and trametinib (T) in patients (pts) with BRAF V600–mutant (mut) melanoma brain metastases (MBM). J Clin Oncol. 2017;35:9506–6.
•• Margolin K, Ernstoff MS, Hamid O, Lawrence D, McDermott D, Puzanov I, et al. Ipilimumab in patients with melanoma and brain metastases: an open-label, phase 2 trial. Lancet Oncol. 2012;13:459–65. This phase 2 trial of ipilimumab was the first prospective study to report intracranial activity of immune checkpoint inhibitors in patients with brain metastases from melanoma. The study included patients with both symptomatic and asymptomatic brain metastases.
Brastianos PK, Carter SL, Santagata S, Cahill DP, Taylor-Weiner A, Jones RT, et al. Genomic characterization of brain metastases reveals branched evolution and potential therapeutic targets. Cancer Discov. 2015;5:1164–77.
Gooden MJM, de Bock GH, Leffers N, Daemen T, Nijman HW. The prognostic influence of tumour-infiltrating lymphocytes in cancer: a systematic review with meta-analysis. Br J Cancer. 2011;105:93–103.
Koelzer VH, Rothschild SI, Zihler D, Wicki A, Willi B, Willi N, et al. Systemic inflammation in a melanoma patient treated with immune checkpoint inhibitors—an autopsy study. J Immunother Cancer. 2016;4:13.
Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–23.
Eggermont AMM, Chiarion-Sileni V, Grob J-J, Dummer R, Wolchok JD, Schmidt H, et al. Adjuvant ipilimumab versus placebo after complete resection of high-risk stage III melanoma (EORTC 18071): a randomised, double-blind, phase 3 trial. Lancet Oncol. 2015;16:522–30.
Powles T, O’Donnell PH, Massard C, Arkenau H-T, Friedlander TW, Hoimes C, et al. Updated efficacy and tolerability of durvalumab in locally advanced or metastatic urothelial carcinoma. J Clin Oncol. 2017;35:286–6.
Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, et al. Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N Engl J Med. 2017;377:1919–29.
Parakh S, Park JJ, Mendis S, Rai R, Xu W, Lo S, et al. Efficacy of anti-PD-1 therapy in patients with melanoma brain metastases. Br J Cancer. 2017;116:1558–63.
•• Di Giacomo AM, Ascierto PA, Queirolo P, Pilla L, Ridolfi R, Santinami M, et al. Three-year follow-up of advanced melanoma patients who received ipilimumab plus fotemustine in the Italian Network for Tumor Biotherapy (NIBIT)-M1 phase II study. Ann Oncol Off J Eur Soc Med Oncol. 2015;26:798–803. The phase 2 study of ipilimumab plus fotemustine included patients with asymptomatic brain metastases, and reported intracranial activity with the combination.
• Williams NL, Wuthrick EJ, Kim H, Palmer JD, Garg S, Eldredge-Hindy H, et al. Phase 1 study of ipilimumab combined with whole brain radiation therapy or radiosurgery for melanoma patients with brain metastases. Int J Radiat Oncol Biol Phys. 2017;99:22–30. This early phase study showed that ipilimumab can be safely combined with stereotactic radiosurgery and whole brain radiation therapy.
•• Long GV, Atkinson V, Menzies AM, Lo S, Guminski AD, Brown MP, et al. A randomized phase II study of nivolumab or nivolumab combined with ipilimumab in patients (pts) with melanoma brain metastases (mets): the Anti-PD1 Brain Collaboration (ABC). J Clin Oncol. 2017;35:9508–8. In this ongoing phase 2 clinical trial, treatment naïve melanoma patients with asymptomatic brain metastases were randomized to receive either a combination of ipilimumab and nivolumab or nivolumab alone. A third arm included symptomatic melanoma brain metastases patients treated with single agent nivolumab.
•• Goldberg SB, Gettinger SN, Mahajan A, Chiang AC, Herbst RS, Sznol M, et al. Pembrolizumab for patients with melanoma or non-small-cell lung cancer and untreated brain metastases: early analysis of a non-randomised, open-label, phase 2 trial. Lancet Oncol. 2016;17:976–83. This prospective phase 2 clinical trial is evaluating the role of pembrolizumab in the management of brain metastases from melanoma and non-small cell lung cancer.
•• Tawbi HA-H, Forsyth PAJ, Algazi AP, Hamid O, Hodi FS, Moschos SJ, et al. Efficacy and safety of nivolumab (NIVO) plus ipilimumab (IPI) in patients with melanoma (MEL) metastatic to the brain: Results of the phase II study CheckMate 204. J Clin Oncol. 2017;35:9507–7. The preliminary results from the ongoing phase 2 clinical trial of nivolumab plus ipilimumab showed encouraging response rates in patients with asymptomatic brain metastases from melanoma.
Ngwa W, Irabor OC, Schoenfeld JD, Hesser J, Demaria S, Formenti SC. Using immunotherapy to boost the abscopal effect. Nat Rev Cancer. 2018;18:313–22.
Grass GD, Krishna N, Kim S. The immune mechanisms of abscopal effect in radiation therapy. Curr Probl Cancer. 2016;40:10–24.
Barker HE, Paget JTE, Khan AA, Harrington KJ. The tumour microenvironment after radiotherapy: mechanisms of resistance and recurrence. Nat Rev Cancer. 2015;15:409–25.
Kiess AP, Wolchok JD, Barker CA, Postow MA, Tabar V, Huse JT, et al. Stereotactic radiosurgery for melanoma brain metastases in patients receiving ipilimumab: safety profile and efficacy of combined treatment. Int J Radiat Oncol Biol Phys. 2015;92:368–75.
Skrepnik T, Sundararajan S, Cui H, Stea B. Improved time to disease progression in the brain in patients with melanoma brain metastases treated with concurrent delivery of radiosurgery and ipilimumab. Oncoimmunology. 2017;6:e1283461.
• Qian JM, Yu JB, Kluger HM, Chiang VLS. Timing and type of immune checkpoint therapy affect the early radiographic response of melanoma brain metastases to stereotactic radiosurgery. Cancer. 2016;122:3051–8. This retrospective single institution study assessed the impact of the timing of immune checkpoint inhibitor in relation to stereotactic radiosurgery and concluded that immunotherapy within 4 weeks of stereotactic radiosurgery provided better intracranial response.
Liniker E, Menzies AM, Kong BY, Cooper A, Ramanujam S, Lo S, et al. Activity and safety of radiotherapy with anti-PD-1 drug therapy in patients with metastatic melanoma. Oncoimmunology. 2016;5:e1214788.
Postow MA, Sidlow R, Hellmann MD. Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med. 2018;378:158–68.
Tivol EA, Borriello F, Schweitzer AN, Lynch WP, Bluestone JA, Sharpe AH. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity. 1995;3:541–7.
Waterhouse P, Penninger JM, Timms E, Wakeham A, Shahinian A, Lee KP, et al. Lymphoproliferative disorders with early lethality in mice deficient in Ctla-4. Science. 1995;270:985–8.
Nishimura H, Nose M, Hiai H, Minato N, Honjo T. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity. 1999;11:141–51.
Abdel-Rahman O, ElHalawani H, Fouad M. Risk of endocrine complications in cancer patients treated with immune check point inhibitors: a meta-analysis. Future Oncol Lond Engl. 2016;12:413–25.
Naidoo J, Wang X, Woo KM, Iyriboz T, Halpenny D, Cunningham J, et al. Pneumonitis in patients treated with anti-programmed Death-1/programmed death ligand 1 therapy. J Clin Oncol Off J Am Soc Clin Oncol. 2017;35:709–17.
Morganstein DL, Lai Z, Spain L, Diem S, Levine D, Mace C, et al. Thyroid abnormalities following the use of cytotoxic T-lymphocyte antigen-4 and programmed death receptor protein-1 inhibitors in the treatment of melanoma. Clin Endocrinol (Oxf). 2017;86:614–20.
Osorio JC, Ni A, Chaft JE, Pollina R, Kasler MK, Stephens D, et al. Antibody-mediated thyroid dysfunction during T-cell checkpoint blockade in patients with non-small-cell lung cancer. Ann Oncol Off J Eur Soc Med Oncol. 2017;28:583–9.
Iwama S, De Remigis A, Callahan MK, Slovin SF, Wolchok JD, Caturegli P. Pituitary expression of CTLA-4 mediates hypophysitis secondary to administration of CTLA-4 blocking antibody. Sci Transl Med. 2014;6:230ra45.
Byrne EH, Fisher DE. Immune and molecular correlates in melanoma treated with immune checkpoint blockade. Cancer. 2017;123:2143–53.
Esfahani K, Miller WH. Reversal of autoimmune toxicity and loss of tumor response by interleukin-17 blockade. N Engl J Med. 2017;376:1989–91.
Gauvain C, Vauléon E, Chouaid C, Lerhun E, Jabot L, Scherpereel A, et al. Intracerebral efficacy and tolerance of nivolumab in non-small-cell lung cancer patients with brain metastases. Lung Cancer Amst Neth. 2018;116:62–6.
Weber JS, Hodi FS, Wolchok JD, Topalian SL, Schadendorf D, Larkin J, et al. Safety profile of nivolumab monotherapy: a pooled analysis of patients with advanced melanoma. J Clin Oncol Off J Am Soc Clin Oncol. 2017;35:785–92.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Adam Lauko, Bicky Thapa, and Vyshak Alva Venur declare no conflict of interest.
Manmeet S. Ahluwalia has received grants from Tracon and Novartis, personal fees from Monteris Medical, Caris Life Sciences, MRI Solutions, CBT Pharmaceuticals, Flatiron, Varian, and VBI vaccines, personal fees and grants from Elekta, Incyte, BMS, AstraZeneca, Novocur, and Abbvie, and has received personal fees and has stock options in Mimivax.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Neuro-oncology
Rights and permissions
About this article
Cite this article
Lauko, A., Thapa, B., Venur, V.A. et al. Management of Brain Metastases in the New Era of Checkpoint Inhibition. Curr Neurol Neurosci Rep 18, 70 (2018). https://doi.org/10.1007/s11910-018-0877-8
Published:
DOI: https://doi.org/10.1007/s11910-018-0877-8