Abstract
Purpose of Review
Biofilm represents an organized structure of microorganisms within an extracellular matrix attached to a surface. While the importance of biofilm in prosthetic heart valve and catheter-related infections has been known since the 1980s, the role of mucosal biofilm in human disease pathogenesis has only recently been elucidated. It is now clear that mucosal biofilm is present in both healthy and pathologic states. The purpose of this review is to examine the role of mucosal biofilm in pediatric respiratory infections.
Recent Findings
Mucosal biofilm has been implicated in relationship to several pediatric respiratory infections, including tonsillitis, adenoiditis, otitis media with effusion, chronic rhinosinusitis, persistent endobronchial infection, and bronchiectasis. In these conditions, core pathogens are detected in the biofilm, biofilm organisms are often detected by molecular techniques when conventional cultures are negative, and biofilm presence is more extensive in relation to disease than in healthy tissues. In chronic rhinosinusitis, the presence of polymicrobial biofilm is also a predictor of poorer outcome following sinus surgery. Biofilm in the tonsillar and adenoidal compartments plays a distinct role in contributing to disease in the middle ear and sinuses.
Summary
Key observations regarding the relevance of biofilm to pediatric respiratory infections include (1) the association between the presence of biofilm and persistent/recurrent and more severe disease in these tissues despite antibiotic treatment, (2) linkage between biofilm core pathogens and acute infections, and (3) interrelationship between biofilm presence in one tissue and persistent or recurrent infection in an adjacent tissue. A greater understanding of the significance of mucosal biofilm will undoubtedly emerge with the development of effective means of eradicating mucosal biofilm.


Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Parsek MR, Singh PK. Bacterial biofilms: an emerging link to disease pathogenesis. Annu Rev Microbiol. 2003;57:677–701.
Ramakrishnan Y, Shields RC, Elbadawey MR, Wilson JA. Biofilms in chronic rhinosinusitis: what is new and where next? J Laryngol Otol. 2015;129(8):744–51.
Donlan RM, Costerton JW. Biofilms: survival mechanisms of clinically relevant microorganisms. Clin Microbiol Rev. 2002;15(2):167–93.
Chiang WC, Pamp SJ, Nilsson M, Givskov M, Tolker-Nielsen T. The metabolically active subpopulation in Pseudomonas aeruginosa biofilms survives exposure to membrane-targeting antimicrobials via distinct molecular mechanisms. FEMS Immunol Med Microbiol. 2012;65(2):245–56.
Hoyle BD, Costerton JW. Bacterial resistance to antibiotics: the role of biofilms. Prog Drug Res. 1991;37:91–105.
Skariyachan S, Sridhar VS, Packirisamy S, Kumargowda ST, Challapilli SB. Recent perspectives on the molecular basis of biofilm formation by Pseudomonas aeruginosa and approaches for treatment and biofilm dispersal. Folia Microbiol. 2018.
Nickel JC, Ruseska I, Wright JB, Costerton JW. Tobramycin resistance of Pseudomonas aeruginosa cells growing as a biofilm on urinary catheter material. Antimicrob Agents Chemother. 1985;27(4):619–24.
Jefferson KK. What drives bacteria to produce a biofilm? FEMS Microbiol Lett. 2004;236(2):163–73.
Chew SC, Kundukad B, Seviour T, van der Maarel JR, Yang L, Rice SA, et al. Dynamic remodeling of microbial biofilms by functionally distinct exopolysaccharides. MBio. 2014;5(4):e01536–14.
Stoodley P, Sauer K, Davies DG, Costerton JW. Biofilms as complex differentiated communities. Annu Rev Microbiol. 2002;56:187–209.
•• Koo H, Allan RN, Howlin RP, Stoodley P, Hall-Stoodley L. Targeting microbial biofilms: current and prospective therapeutic strategies. Nat Rev Microbiol. 2017;15(12):740–55. This is an excellent review of stages of biofilm formation and approaches toward targeting biofilm of specific pathogens.
• Domenech M, Ruiz S, Moscoso M, Garcia E. In vitro biofilm development of Streptococcus pneumoniae and formation of choline-binding protein-DNA complexes. Environ Microbiol Rep. 2015;7(5):715–27. This study exam is the role of extracellular DNA in Streptococcus pneumonia biofilm formation.
Manzenreiter R, Kienberger F, Marcos V, Schilcher K, Krautgartner WD, Obermayer A, et al. Ultrastructural characterization of cystic fibrosis sputum using atomic force and scanning electron microscopy. J Cyst Fibros. 2012;11(2):84–92.
Draghi F, Bortolotto C. Intersection syndrome: ultrasound imaging. Skelet Radiol. 2014;43(3):283–7.
• Hong W, Juneau RA, Pang B, Swords WE. Survival of bacterial biofilms within neutrophil extracellular traps promotes nontypeable Haemophilus influenzae persistence in the chinchilla model for otitis media. J Innate Immun. 2009;1(3):215–24. This study demonstrates how neutrophil extracellular traps (NETs) promote persistence of Haemophilus influenza biofilm.
Goldmann O, Medina E. The expanding world of extracellular traps: not only neutrophils but much more. Front Immunol. 2012;3:420.
• Goodman SD, Obergfell KP, Jurcisek JA, Novotny LA, Downey JS, Ayala EA, et al. Biofilms can be dispersed by focusing the immune system on a common family of bacterial nucleoid-associated proteins. Mucosal Immunol. 2011;4(6):625–37. This study demonstrates a therapeutic strategy for dispersal of biofilm formation by focusing on bacterial nucleoid associated proteins.
Dorman CJ. Nucleoid-associated proteins and bacterial physiology. Adv Appl Microbiol. 2009;67:47–64.
Devaraj A, Buzzo J, Rocco CJ, Bakaletz LO, Goodman SD. The DNABII family of proteins is comprised of the only nucleoid associated proteins required for nontypeable Haemophilus influenzae biofilm structure. MicrobiologyOpen. 2017.
• Pamp SJ, Tolker-Nielsen T. Multiple roles of biosurfactants in structural biofilm development by Pseudomonas aeruginosa. J Bacteriol. 2007;189(6):2531–9. This study discusses potential biofilm-promoting and biofilm-inhibiting properties of biosurfactants.
Prince AA, Steiger JD, Khalid AN, Dogrhamji L, Reger C, Eau Claire S, et al. Prevalence of biofilm-forming bacteria in chronic rhinosinusitis. Am J Rhinol. 2008;22(3):239–45.
Khosravi Y, Ling LC, Loke MF, Shailendra S, Prepageran N, Vadivelu J. Determination of the biofilm formation capacity of bacterial pathogens associated with otorhinolaryngologic diseases in the Malaysian population. Eur Arch Otorhinolaryngol. 2014;271(5):1227–33.
Foreman A, Wormald PJ. Different biofilms, different disease? A clinical outcomes study. Laryngoscope. 2010;120(8):1701–6.
Stephenson MF, Mfuna L, Dowd SE, Wolcott RD, Barbeau J, Poisson M, et al. Molecular characterization of the polymicrobial flora in chronic rhinosinusitis. J Otolaryngol Head Neck Surg. 2010;39(2):182–7.
Zhang Z, Kofonow JM, Finkelman BS, Doghramji L, Chiu AG, Kennedy DW, et al. Clinical factors associated with bacterial biofilm formation in chronic rhinosinusitis. Otolaryngology--head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery. 2011;144(3):457–62.
• Cope EK, Goldstein-Daruech N, Kofonow JM, Christensen L, McDermott B, Monroy F, et al. Regulation of virulence gene expression resulting from Streptococcus pneumoniae and nontypeable Haemophilus influenzae interactions in chronic disease. PloS One. 2011;6(12):e28523. This study examines regulation of virulence gene expression in biofilms.
Hoa M, Tomovic S, Nistico L, Hall-Stoodley L, Stoodley P, Sachdeva L, et al. Identification of adenoid biofilms with middle ear pathogens in otitis-prone children utilizing SEM and FISH. Int J Pediatr Otorhinolaryngol. 2009;73(9):1242–8.
Mahajan A, Singh B, Kashyap D, Kumar A, Mahajan P. Interspecies communication and periodontal disease. TheScientificWorldJOURNAL. 2013;2013:765434.
Mladina R, Skitarelic N, Music S, Ristic M. A biofilm exists on healthy mucosa of the paranasal sinuses: a prospectively performed, blinded, scanning electron microscope study. Clin Otolaryngol. 2010;35(2):104–10.
Hamilos DL. Host-microbial interactions in patients with chronic rhinosinusitis. J Allergy Clin Immunol. 2014;133(3):640–53 e4.
• Abreu NA, Nagalingam NA, Song Y, Roediger FC, Pletcher SD, Goldberg AN, et al. Sinus microbiome diversity depletion and Corynebacterium tuberculostearicum enrichment mediates rhinosinusitis. Sci Transl Med. 2012;4(151):151ra24. This study introduces the concept of dysbiosis as a pathologic feature and important disease-promoting mechanism in of chronic rhinosinusitis.
De Groote MA, Fang FC. NO inhibitions: antimicrobial properties of nitric oxide. Clin Infect Dis. 1995;21(Suppl 2):S162–5.
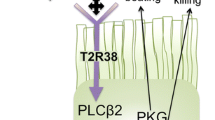
Yan CH, Hahn S, McMahon D, Bonislawski D, Kennedy DW, Adappa ND, et al. Nitric oxide production is stimulated by bitter taste receptors ubiquitously expressed in the sinonasal cavity. Am J Rhinol Allergy. 2017;31(2):85–92.
Lundberg JO. Nitric oxide and the paranasal sinuses. Anat Rec (Hoboken). 2008;291(11):1479–84.
Lee RJ, Xiong G, Kofonow JM, Chen B, Lysenko A, Jiang P, et al. T2R38 taste receptor polymorphisms underlie susceptibility to upper respiratory infection. J Clin Invest. 2012;122(11):4145–59.
Walker WT, Jackson CL, Allan RN, Collins SA, Kelso MJ, Rineh A, et al. Primary ciliary dyskinesia ciliated airway cells show increased susceptibility to Haemophilus influenzae biofilm formation. Eur Respir J. 2017;50(3).
Pifferi M, Bush A, Caramella D, Di Cicco M, Zangani M, Chinellato I, et al. Agenesis of paranasal sinuses and nasal nitric oxide in primary ciliary dyskinesia. Eur Respir J. 2011;37(3):566–71.
Krantz C, Janson C, Hollsing A, Alving K, Malinovschi A. Exhaled and nasal nitric oxide in relation to lung function, blood cell counts and disease characteristics in cystic fibrosis. J Breath Res. 2017;11(2):026001.
Shapiro AJ, Josephson M, Rosenfeld M, Yilmaz O, Davis SD, Polineni D, et al. Accuracy of nasal nitric oxide measurement as a diagnostic test for primary ciliary dyskinesia. A systematic review and meta-analysis. Ann Am Thorac Soc. 2017;14(7):1184–96.
Adappa ND, Truesdale CM, Workman AD, Doghramji L, Mansfield C, Kennedy DW, et al. Correlation of T2R38 taste phenotype and in vitro biofilm formation from nonpolypoid chronic rhinosinusitis patients. Int Forum Allergy Rhinol. 2016;6(8):783–91.
Psaltis AJ, Wormald PJ, Ha KR, Tan LW. Reduced levels of lactoferrin in biofilm-associated chronic rhinosinusitis. Laryngoscope. 2008;118(5):895–901.
Singh PK, Parsek MR, Greenberg EP, Welsh MJ. A component of innate immunity prevents bacterial biofilm development. Nature. 2002;417(6888):552–5.
Deziel E, Comeau Y, Villemur R. Initiation of biofilm formation by Pseudomonas aeruginosa 57RP correlates with emergence of hyperpiliated and highly adherent phenotypic variants deficient in swimming, swarming, and twitching motilities. J Bacteriol. 2001;183(4):1195–204.
• Ahmad S, Tyrrell J, Walton WG, Tripathy A, Redinbo MR, Tarran R. Short palate, lung, and nasal epithelial clone 1 has antimicrobial and antibiofilm activities against the Burkholderia cepacia complex. Antimicrob Agents Chemother. 2016;60(10):6003–12. The study discusses the anti-biofilm properties of SPLUNC1 toward Burkholderia cepacia complex biofilm.
• Liu Y, Bartlett JA, Di ME, Bomberger JM, Chan YR, Gakhar L, et al. SPLUNC1/BPIFA1 contributes to pulmonary host defense against Klebsiella pneumoniae respiratory infection. Am J Pathol. 2013;182(5):1519–31. The study discusses the anti-biofilm properties of SPLUNC1 toward Klebsiella pneumonia biofilm.
• Gakhar L, Bartlett JA, Penterman J, Mizrachi D, Singh PK, Mallampalli RK, et al. PLUNC is a novel airway surfactant protein with anti-biofilm activity. PloS One. 2010;5(2):e9098 The study discusses the anti-biofilm properties of SPLUNC1.
• Liu Y, Di ME, Chu HW, Liu X, Wang L, Wenzel S, et al. Increased susceptibility to pulmonary Pseudomonas infection in Splunc1 knockout mice. J Immunol. 2013;191(8):4259–68. The study discusses the anti-biofilm properties of SPLUNC1 toward Pseudomonas biofilm.
Mireles JR 2nd, Toguchi A, Harshey RM. Salmonella enterica serovar typhimurium swarming mutants with altered biofilm-forming abilities: surfactin inhibits biofilm formation. J Bacteriol. 2001;183(20):5848–54.
Walencka E, Rozalska S, Sadowska B, Rozalska B. The influence of Lactobacillus acidophilus-derived surfactants on staphylococcal adhesion and biofilm formation. Folia Microbiol. 2008;53(1):61–6.
• Tarran R, Redinbo MR. Mammalian short palate lung and nasal epithelial clone 1 (SPLUNC1) in pH-dependent airway hydration. Int J Biochem Cell Biol. 2014;52:130–5. This study demonstrates the importance of SPLUNC1 in pH dependent airway hydration and its relevance to cystic fibrosis.
Garland AL, Walton WG, Coakley RD, Tan CD, Gilmore RC, Hobbs CA, et al. Molecular basis for pH-dependent mucosal dehydration in cystic fibrosis airways. Proc Natl Acad Sci U S A. 2013;110(40):15973–8.
Bingle L, Barnes FA, Cross SS, Rassl D, Wallace WA, Campos MA, et al. Differential epithelial expression of the putative innate immune molecule SPLUNC1 in cystic fibrosis. Respir Res. 2007;8:79.
• Tsou YA, Chen CM, Lin TC, Hu FW, Tai CJ, Chen HC, et al. Decreased SPLUNC1 expression is associated with Pseudomonas infection in surgically treated chronic rhinosinusitis patients who may require repeated sinus surgery. Laryngoscope. 2013;123(4):845–51. This study found a relationship between decreased sinus tissue SPLUNC1 expression and Pseudomonas infection in surgically treated chronic rhinosinusitis patients who require repeated sinus surgery.
Benincasa M, Mattiuzzo M, Herasimenka Y, Cescutti P, Rizzo R, Gennaro R. Activity of antimicrobial peptides in the presence of polysaccharides produced by pulmonary pathogens. J Pept Sci. 2009;15(9):595–600.
• Jones EA, McGillivary G, Bakaletz LO. Extracellular DNA within a nontypeable Haemophilus influenzae-induced biofilm binds human beta defensin-3 and reduces its antimicrobial activity. J Innate Immun. 2013;5(1):24–38. The study demonstrates that extracellular DNA from nontypeable Haemophilus influenza induced biofilm can neutralize the antimicrobial activity of human beta defensin-3.
Leid JG, Shirtliff ME, Costerton JW, Stoodley P. Human leukocytes adhere to, penetrate, and respond to Staphylococcus aureus biofilms. Infect Immun. 2002;70(11):6339–45.
Stoodley P, Lewandowski Z, Boyle JD, Lappin-Scott HM. Structural deformation of bacterial biofilms caused by short-term fluctuations in fluid shear: an in situ investigation of biofilm rheology. Biotechnol Bioeng. 1999;65(1):83–92.
• Leid JG, Willson CJ, Shirtliff ME, Hassett DJ, Parsek MR, Jeffers AK. The exopolysaccharide alginate protects Pseudomonas aeruginosa biofilm bacteria from IFN-gamma-mediated macrophage killing. J Immunol. 2005;175(11):7512–8. This study demonstrates importance of alginate in Pseudomonas biofilm formation.
Hall-Stoodley L, Stoodley P. Evolving concepts in biofilm infections. Cell Microbiol. 2009;11(7):1034–43.
Hochstim CJ, Choi JY, Lowe D, Masood R, Rice DH. Biofilm detection with hematoxylin-eosin staining. Arch Otolaryngol Head Neck Surg. 2010;136(5):453–6.
Ha KR, Psaltis AJ, Tan L, Wormald PJ. A sheep model for the study of biofilms in rhinosinusitis. Am J Rhinol. 2007;21(3):339–45.
Foreman A, Singhal D, Psaltis AJ, Wormald PJ. Targeted imaging modality selection for bacterial biofilms in chronic rhinosinusitis. Laryngoscope. 2010;120(2):427–31.
Psaltis AJ, Ha KR, Beule AG, Tan LW, Wormald PJ. Confocal scanning laser microscopy evidence of biofilms in patients with chronic rhinosinusitis. Laryngoscope. 2007;117(7):1302–6.
Stepanovic S, Vukovic D, Dakic I, Savic B, Svabic-Vlahovic M. A modified microtiter-plate test for quantification of staphylococcal biofilm formation. J Microbiol Methods. 2000;40(2):175–9.
Jurcisek JA, Dickson AC, Bruggeman ME, Bakaletz LO. In vitro biofilm formation in an 8-well chamber slide. J Vis Exp 2011(47).
Clement S, Vaudaux P, Francois P, Schrenzel J, Huggler E, Kampf S, et al. Evidence of an intracellular reservoir in the nasal mucosa of patients with recurrent Staphylococcus aureus rhinosinusitis. J Infect Dis. 2005;192(6):1023–8.
Plouin-Gaudon I, Clement S, Huggler E, Chaponnier C, Francois P, Lew D, et al. Intracellular residency is frequently associated with recurrent Staphylococcus aureus rhinosinusitis. Rhinology. 2006;44(4):249–54.
Corriveau MN, Zhang N, Holtappels G, Van Roy N, Bachert C. Detection of Staphylococcus aureus in nasal tissue with peptide nucleic acid-fluorescence in situ hybridization. Am J Rhinol Allergy. 2009;23(5):461–5.
Tan NC, Foreman A, Jardeleza C, Douglas R, Tran H, Wormald PJ. The multiplicity of Staphylococcus aureus in chronic rhinosinusitis: correlating surface biofilm and intracellular residence. Laryngoscope. 2012;122(8):1655–60.
Tan NC, Foreman A, Jardeleza C, Douglas R, Vreugde S, Wormald PJ. Intracellular Staphylococcus aureus: the Trojan horse of recalcitrant chronic rhinosinusitis? Int Forum Allergy Rhinol. 2013;3(4):261–6.
Chole RA, Faddis BT. Anatomical evidence of microbial biofilms in tonsillar tissues: a possible mechanism to explain chronicity. Arch Otolaryngol Head Neck Surg. 2003;129(6):634–6.
Galli J, Ardito F, Calo L, Mancinelli L, Imperiali M, Parrilla C, et al. Recurrent upper airway infections and bacterial biofilms. J Laryngol Otol. 2007;121(4):341–4.
Galli J, Calo L, Ardito F, Imperiali M, Bassotti E, Fadda G, et al. Biofilm formation by Haemophilus influenzae isolated from adeno-tonsil tissue samples, and its role in recurrent adenotonsillitis. Acta Otorhinolaryngol Ital. 2007;27(3):134–8.
Galli J, Calo L, Ardito F, Imperiali M, Passali GC, Carnevale N, et al. Bacterial biofilm identification in the rhinopharingeal mucosa of children with recurrent infection of the upper respiratory tract and otitis media. La Pediatria medica e chirurgica : Medical and surgical pediatrics. 2008;30(1):31–4. Identificazione di biofilm batterici nella mucosa rinofaringea di bambini affetti da infezioni ricorrenti delle vie aeree superiori associate ad otite media.
Torretta S, Drago L, Marchisio P, Gaffuri M, Clemente IA, Pignataro L. Topographic distribution of biofilm-producing bacteria in adenoid subsites of children with chronic or recurrent middle ear infections. Ann Otol Rhinol Laryngol. 2013;122(2):109–13.
• Coticchia J, Zuliani G, Coleman C, Carron M, Gurrola J 2nd, Haupert M, et al. Biofilm surface area in the pediatric nasopharynx: chronic rhinosinusitis vs obstructive sleep apnea. Arch Otolaryngol Head Neck Surg. 2007;133(2):110–4. This study demonstrates an association between adenoidal biofilm and pediatric chronic rhinosinusitis.
Lee D, Rosenfeld RM. Adenoid bacteriology and sinonasal symptoms in children. Otolaryngology--head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery. 1997;116(3):301–7.
Muntz HR, Lusk RP. Bacteriology of the ethmoid bullae in children with chronic sinusitis. Arch Otolaryngol Head Neck Surg. 1991;117(2):179–81.
Hall-Stoodley L, Hu FZ, Gieseke A, Nistico L, Nguyen D, Hayes J, et al. Direct detection of bacterial biofilms on the middle-ear mucosa of children with chronic otitis media. JAMA. 2006;296(2):202–11.
Post JC, Hiller NL, Nistico L, Stoodley P, Ehrlich GD. The role of biofilms in otolaryngologic infections: update 2007. Curr Opin Otolaryngol Head Neck Surg. 2007;15(5):347–51.
Van Hoecke H, De Paepe AS, Lambert E, Van Belleghem JD, Cools P, Van Simaey L, et al. Haemophilus influenzae biofilm formation in chronic otitis media with effusion. Eur Arch Otorhinolaryngol. 2016;273(11):3553–60.
Cryer J, Schipor I, Perloff JR, Palmer JN. Evidence of bacterial biofilms in human chronic sinusitis. ORL J Otorhinolaryngol Relat Spec. 2004;66(3):155–8.
Ferguson BJ, Stolz DB. Demonstration of biofilm in human bacterial chronic rhinosinusitis. Am J Rhinol. 2005;19(5):452–7.
Sanclement JA, Webster P, Thomas J, Ramadan HH. Bacterial biofilms in surgical specimens of patients with chronic rhinosinusitis. Laryngoscope. 2005;115(4):578–82.
Singhal D, Psaltis AJ, Foreman A, Wormald PJ. The impact of biofilms on outcomes after endoscopic sinus surgery. Am J Rhinol Allergy. 2010;24(3):169–74.
Hochstim CJ, Masood R, Rice DH. Biofilm and persistent inflammation in endoscopic sinus surgery. Otolaryngology--head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery. 2010;143(5):697–8.
Perloff JR, Palmer JN. Evidence of bacterial biofilms on frontal recess stents in patients with chronic rhinosinusitis. Am J Rhinol. 2004;18(6):377–80.
Harvey RJ, Lund VJ. Biofilms and chronic rhinosinusitis: systematic review of evidence, current concepts and directions for research. Rhinology. 2007;45(1):3–13.
Bendouah Z, Barbeau J, Hamad WA, Desrosiers M. Biofilm formation by Staphylococcus aureus and Pseudomonas aeruginosa is associated with an unfavorable evolution after surgery for chronic sinusitis and nasal polyposis. Otolaryngology--head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery. 2006;134(6):991–6.
Zernotti ME, Angel Villegas N, Roques Revol M, Baena-Cagnani CE, Arce Miranda JE, Paredes ME, et al. Evidence of bacterial biofilms in nasal polyposis. J Investig Allergol Clin Immunol. 2010;20(5):380–5.
Motta JP, Flannigan KL, Agbor TA, Beatty JK, Blackler RW, Workentine ML, et al. Hydrogen sulfide protects from colitis and restores intestinal microbiota biofilm and mucus production. Inflamm Bowel Dis. 2015;21(5):1006–17.
Dickson RP, Erb-Downward JR, Prescott HC, Martinez FJ, Curtis JL, Lama VN, et al. Cell-associated bacteria in the human lung microbiome. Microbiome. 2014;2:28.
Cope EK, Lynch SV. Novel microbiome-based therapeutics for chronic rhinosinusitis. Curr Allergy Asthma Rep. 2015;15(3):504.
Jardeleza C, Miljkovic D, Baker L, Boase S, Tan NC, Koblar SA, et al. Inflammasome gene expression alterations in Staphylococcus aureus biofilm-associated chronic rhinosinusitis. Rhinology. 2013;51(4):315–22.
Drilling A, Coombs GW, Tan HL, Pearson JC, Boase S, Psaltis A, et al. Cousins, siblings, or copies: the genomics of recurrent Staphylococcus aureus infections in chronic rhinosinusitis. Int Forum Allergy Rhinol. 2014;4(12):953–60.
Ou J, Drilling A, Singhal D, Tan NC, Wallis-Hill D, Vreugde S, et al. Association of intracellular Staphylococcus aureus with prognosis in chronic rhinosinusitis. Int Forum Allergy Rhinol. 2016;6(8):792–9.
Brook I, Yocum P, Shah K. Aerobic and anaerobic bacteriology of concurrent chronic otitis media with effusion and chronic sinusitis in children. Arch Otolaryngol Head Neck Surg. 2000;126(2):174–6.
Davcheva-Chakar M, Kaftandzhieva A, Zafirovska B. Adenoid Vegetations - reservoir of bacteria for chronic otitis media with effusion and chronic rhinosinusitis. Pril (Makedon Akad Nauk Umet Odd Med Nauki). 2015;36(3):71–6.
Verhagen LM, de Groot R. Recurrent, protracted and persistent lower respiratory tract infection: a neglected clinical entity. J Infect. 2015;71(Suppl 1):S106–11.
Ishak A, Everard ML. Persistent and recurrent bacterial bronchitis-a paradigm shift in our understanding of chronic respiratory disease. Front Pediatr. 2017;5:19.
Maselli DJ, Amalakuhan B, Keyt H, Diaz AA. Suspecting non-cystic fibrosis bronchiectasis: what the busy primary care clinician needs to know. Int J Clin Pract. 2017;71(2).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Daniel L. Hamilos declares that he has no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by Dr. Hamilos.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Pediatric Infectious Diseases
Rights and permissions
About this article
Cite this article
Hamilos, D.L. Biofilm Formations in Pediatric Respiratory Tract Infection. Curr Infect Dis Rep 21, 6 (2019). https://doi.org/10.1007/s11908-019-0658-9
Published:
DOI: https://doi.org/10.1007/s11908-019-0658-9