Abstract
Purpose of the Review
To describe trends and clinical characteristics of HIV and cerebrovascular disease between 1990 and 2021 in LMICs and identify the gaps in our understanding.
Recent Findings
In the era of antiretroviral therapy (ART), people living with HIV (PLWH) live longer and risk excess cerebrovascular events due to ageing and HIV-driven factors. Despite the highest burden of HIV infection in low-to-middle income countries, there is underreporting in the literature of cerebrovascular events in this population. We systematically reviewed published literature for primary clinical studies in adult PLWH and cerebrovascular disease in LMICs.
Summary
The clinical phenotype of cerebrovascular disease among PLWH over the last three decades in LMICs has evolved and transitioned to an older group with overlapping cerebrovascular risk factors. There is an important need to increase research in this population and standardise reporting to facilitate understanding, guide development of appropriate interventions, and evaluate their impact.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The global roll-out of effective antiretroviral treatment (ART) regimens for people living with HIV (PLWH) has significantly improved life expectancy [1, 2]. As this population ages, it has been observed that other comorbidities such as cerebrovascular disease (CVD) are seen with greater frequency than in the general population [3, 4••, 5, 6, 7••]. CVD can manifest as stroke (affecting large-to-medium sized arterial vessels) or cognitive impairment (among stroke populations, or non-stroke populations with disease of the small vessels leading to lacunar infarcts or microbleeds). On average, 40–50% of survivors of stroke develop some form of cognitive dysfunction, suggesting that vascular cognitive impairment could become the most common precursor to dementia [8].
There are likely to be multiple mechanisms underlying CVD in PLWH, including HIV-associated factors (chronic inflammation, vasculopathy, opportunistic infections, cardioembolism and coagulopathy) [9], in interplay with traditional cardiovascular risk factors, which may be accelerated by HIV infection or occur through the normal ageing process. In addition, some ARTs have an additive role; for example, specific protease inhibitors are associated with hypercholesterolaemia which, in turn, increases CVD risk [4••, 10].
Much of the work elucidating the associations between HIV and CVD has been carried out in populations in high-income countries (HICs). However, the global burden of HIV is centred in low- and middle-income countries (LMICs) [11], with 12% of the global population, seeing 71% of global HIV infection [12]. This is especially true for Sub-Saharan Africa (SSA). Though the mechanisms driving CVD in PLWH in LMICs and HICs overlap, there will likely be significant regional and cultural variations. As examples, hypertension prevalence is higher in LMICs than HICs and there is evidence that this gap is widening [13]. Other CVD risk factors such as drug and alcohol use vary greatly between populations; and populations in LMICs generally have comparatively decreased access to healthcare resources than their HIC counterparts. Aetiopathogenesis of CVD in PLWH must therefore be studied in LMICs specifically.
Previous reviews have highlighted the paucity of evidence on CVD in PLWH in LMICs [14, 15]. This, coupled with the changing epidemiology of stroke in PLWH, and evidence that stroke in PLWH affects younger individuals, is of greater severity and has greater mortality [15] compared to HIV-uninfected populations, must prompt further study if the often already fragile health systems serving these populations are to adapt to and cope with this rising tide of disease.
This systematic review aims to describe trends and clinical characteristics of HIV and CVD between 1990 and 2021 in LMICs and identify the gaps in our understanding which require further elucidation.
Methods
Search Strategy and Selection Criteria
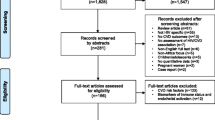
We identified references for this review by searching Medline and PubMed for articles published in English between Jan 1990 and Dec 2021 using the terms, ‘cerebrovascular disorders’ OR ‘stroke’ OR ‘intracranial arteriosclerosis’ OR ‘arteriosclerosis’ OR ‘intracranial embolism’ OR ‘subarachnoid haemorrhage’ OR ‘intracranial haemorrhage’ OR ‘cerebral haemorrhage’ OR ‘vascular disease’ OR ‘vasculitis’ OR ‘CNS vasculitis’ OR ‘vasculopathy’ OR ‘atherosclerosis’ OR ‘cerebral venous thrombosis’ AND ‘human immunodeficiency virus’ OR ‘HIV’ OR ‘ART’ OR ‘PLWH’ AND ‘Asia’ OR ‘South America’ OR ‘Africa’ OR ‘Subsaharan Africa’ OR ‘developing country(ies)’ OR ‘least developed country(ies)’ OR ‘least developed nation(s)’ OR ‘under-developed nation(s)’ OR ‘third world nation(s)’ OR ‘third-world country(ies)’ OR ‘less-developed nation’ OR ‘underdeveloped country’ [term exploded]. The addition of the term ‘AIDS’ did not yield any further publications. Articles were also identified through the reference lists of selected publications and a search of the Cochrane Database. Only articles published in English were included. We excluded reviews if they did not report new primary data, studies limited purely to comparisons of diagnostic techniques, studies on non-cerebrovascular manifestations of HIV, and studies that did not report primary data from an LMIC. LMIC is defined by the world bank as those with <$1085 up to $13205 in per capita gross national income. The inclusion of case reports was limited to Fig. 1.
Data Extraction
Titles and abstracts of each identified manuscript were initially screened for eligibility. Reason for exclusion was recorded for all references not meeting inclusion criteria. Full text manuscripts were then reviewed by a single author for those references thought to meet inclusion criteria based on screening of the title and abstract. For each reference meeting inclusion criteria, we extracted data for the following categories: (1) study year (s) and sites involved, (2) study design, (3) participants number and clinical characteristics, (4) HIV factors [e.g. stage of disease and ART use], (5) comorbidities, (6) aetiology of ischaemic stroke using the Trial of ORG 10172 in Acute Stroke Treatment (TOAST) classification or variations of this, and (5) clinical outcomes such as mortality, severity (e.g. National Institutes of Health Stroke Scale (NIHSS)), and functional status (e.g. modified Rankin Scale (mRS)). Data were abstracted into a pre-specified Microsoft Excel spreadsheet.
Results
Our search returned 329 articles, of which 157 were excluded through screening of the title and abstract, and a further 84 were excluded based on a full-text review of the manuscripts (Fig. 1). Articles were excluded because they were not primary research (n = 33), included non-adult populations (n = 8), were in a language other than English (n = 8), did not originate from LMICs (n = 44), or because HIV and/or cerebrovascular disease was not an exposure or outcome (n = 157). After exclusion, 88 articles were included in the review.
Characteristics of included studies are shown in Table 1. Cross-sectional studies (n = 44, 51%) constituted the majority of included studies, followed by cohort studies (n = 24, 28%), case–control studies (n = 13, 15%), case series (n = 3, 3%), case reports (n = 1) and mixed methods studies (n = 1). Cross-sectional studies ranged from 25 to ~ 42,000 participants with a median sample size of 238, while cohort studies ranged from 26 to ~ 30,000 with a median sample size of 320.
The distribution of included publications by year (Fig. 2A) shows a clear trend toward increasing numbers of publications with time, with a marked increase in the number of publications per year beginning in 2015. This trend has largely been sustained through 2021, with more than five included publications each year since 2015. The largest number of included publications in any year was 11 in 2021. The majority of publications were from Africa (n = 61, 69%) followed by Asia (n = 17, 19%) and South America (n = 10, 11%) (Fig. 2B). Figure 2C shows countries where at least one article was included within LMICs (in black). Of 142 LMICs, only 20 countries (14%) are represented in the included publications.
Participants in the three included case series were people with HIV who experienced strokes in South Africa (n = 2) and Kenya [16••, 17, 18]. Participants in these studies had a mean age in the mid-thirties (32 to 36 years), and the majority had sub-optimally controlled HIV infection. One case series focused on identifying stroke aetiology and found high rates of stroke due to coagulopathies (49%) and meningitis (25%), with the remaining due to cardioembolism (9%) and hypertension (6%) [16••]. The outstanding two case series focused on HIV-associated vasculopathy as a cause of stroke. They found that HIV-associated vasculopathy primarily occurred in PLWH with low CD4 counts and was almost equally likely to present as an occlusive disease (51%) versus aneurysmal disease (49%) [17, 18].
Epidemiology and Clinical Characteristics
Demographics and Epidemiology
Multiple studies described demographic and clinical data specifically for PLWH presenting with CVD, often comparing this with PLWH without CVD, or with HIV-negative CVD sufferers. Three studies reported cognitive function [19, 20••, 21]; the remainder focused on stroke (Table 2). Among these, the mean age of study participants ranged from 29 to 55 years. Between 1990 and 2010, the age range was 29–39 years, but increased to 40–55 years after 2010. Sex distribution across studies was variable, ranging from 9 to 75% female. Between 1990 and 2010, this was 41–75%, and 9–75% after 2010. All studies were hospital-based; four included community and outpatient clinic participants.
Twelve studies assessed the prevalence of HIV in patients presenting with CVD, while others (n = 4) examined the incidence or prevalence of CVD in patients with HIV. Reported incidence rates varied: in Cameroon, Mapoure et al. (2016) estimated a stroke rate of 3 per 1000 person-years; similarly in Taiwan a rate of 2.12 per 1000 person-years was found (Lin et al., 2019); while in Ghana, Sarfo et al. (2021) found a rate of 12.24 per 1000 person-years [22,23,24,25]. Examining stroke prevalence, Brites et al. found a rate of 4.4% among PLWH [26]. The overall hospital prevalence of HIV among stroke admissions in PLWH was between 0.14 and 48% when provided. In contrast, the prevalence of HIV in the CVD population ranged from 2.5 to 48% between 1990 and 2010 and 0.14–8% after 2010.
Our case–control study from Malawi (n = 723) compared hospital-based stroke cases with matched population-based non-stroke controls to estimate the risk of stroke; they found that HIV infection was associated with an increased odds of stroke (adjusted odds ratio [aOR] 3.28, CI [2.05–5.25]) [7]. Moreover, HIV accounted for the second-highest population attributable fraction (15%) overall and the highest among young populations (PAF 42%; age < 45 years) [7].
HIV Factors
The stage of HIV infection during hospital admission was variable across the three decades. The average CD4 T-cell count ranged from 190 to 431 cells/mm3. Between 1990 and 2010, three studies reported advanced disease (i.e. CD4 count < 200 cells/mm3) in 40–53% of cases [27,28,29,30], whereas after 2010, more studies had a median CD4 T-cell count above 200cells/mm3 (Table 2). Viral load was rarely described. Kamtchum-Tatuene et al. reported a median viral load of 1,884 copies/ml, with 55.6% of participants having > 1,000 copies/ml; while in a South African study, 62.5% of patients were not virologically suppressed, despite many patients reporting being on ART [31, 32]. The use of ART was well described, and as shown in Table 2, coverage was generally poor (i.e. < 12%) between 1990 and 2010. After 2010, the ART prevalence substantially increased (22–83%), coinciding with the roll-out of ART programs in these regions.
Only a few studies discussed ART and stroke risk. One of which was ours, we identified an increased stroke risk early in the use of treatment, possibly suggesting an Immune Reconstitution Inflammatory Syndrome (IRIS)-related mechanism [7]. Furthermore, we showed no risk with long-term ART use, and speculated that this might have been underpowered or affected by competing risks. One case report described an ischaemic stroke as a paradoxical IRIS reaction [33]. Some studies corroborated previous findings that protease inhibitors (PIs) increase rates of dyslipidaemia, and Juma et al. found that nucleotide reverse transcriptase inhibitor (NRTI)-based regimens were associated with raised total cholesterol [34, 35].
Cerebrovascular Disease (CVD) Risk Factors
Multiple authors examined traditional cardiovascular risk factors in PLWH. High rates of hypertension were described in many of these studies, particularly those completed more recently. Hypertension prevalence between 1990 and 2010 was 6–11%, and after 2010, this increased to 10–72%. However, studies comparing PLWH to aged-matched HIV-negative stroke patients generally showed no significant difference between the most common cardiovascular risk factors, including hypertension, diabetes mellitus and dyslipidaemia [7, 36], while one study from Zambia found significantly lower rates of traditional CVD risk factors among PLWH compared to HIV-uninfected adults with stroke [37].
Scoring systems for CVD risk were infrequently assessed in studies of stroke. Kuate et al. found that the Framingham score correlated poorly with stroke risk in PLWH, with 67.4% of patients given a low-risk score, likely underestimating overall risk [38].
Carotid disease is a surrogate of CVD. Although there was heterogeneity regarding the definition of carotid disease across studies, those limited to an extracranial evaluation reported a prevalence of carotid disease ranging between 0 and 24%.
Aetiology and Outcome in PLWH and Cerebrovascular Disease
Aetiology
Eight out of 53 (15%) eligible articles reported on the aetiology and/or stroke outcome in PLWH. Prevalence of CT or MRI brain imaging performed in a selected or unselected cohort varied from 87 to 100%. This high uptake was consistent across two decades. The prevalence of ischaemic stroke was higher (57–96%) compared with intracerebral haemorrhage (4–33%; Table 3). The TOAST classification and its variations were used to provide a template to describe the aetiology. Crucially, less than the minimum set of investigations, as agreed in a consensus statement on HIV and stroke, were performed, thus precluding accurate attribution of stroke aetiology [39]. For example, approximately 60% of study participants had CSF to investigate opportunistic infections, and only 60% had an electrocardiogram or echocardiogram looking for a cardioembolic source. Common aetiologies included large vessel vasculopathy (20–37%) and opportunistic infections (5–37%). Twenty per cent of HIV-associated ischaemic stroke was attributed to opportunistic infection in one study [40]. Likewise, Tipping et al. found that a third of PLWH with stroke had evidence of intercurrent opportunistic infections [29]. These study populations had low CD4 T-cell count [29, 40]. In other studies, tuberculous and cryptococcal meningitis and CMV encephalitis were associated with increased stroke risk. Cardioembolic stroke was low in frequency (6–8%) [41,42,43].
Outcome
Six studies reported on hospital mortality and showed high rates (17–21%) Table 3 [20, 29, 37, 38, 40]. However, Heikinheimo et al. showed that death at 6 weeks was higher in the HIV-negative adults with stroke [HIV-negative mortality: 23%, mortality in PLWH and stroke: 18%], but when accounting for age, there was no significant difference in mortality [36]. Furthermore, the functional outcome at 6 weeks was significantly better in PLWH [mRS of 4–5: 32% of HIV-negative and 14% of PLWH (p = 0.015)]. Risk factors for increased mortality included low GCS on admission (p = 0.046), fever during hospitalization (p = 0.003) and hypertension (p = 0.04) [37].
There were limited reports on cognitive outcome, one study described cognitive function and demonstrated the involvement of frontal system syndromes (4/22; 18%) and aphasia (10/22; 45%) compared to the control group (frontal system syndrome 3/22 (14%) and aphasia 8/22(36%)), and another looked at cerebral vasoreactivity in PLWH and correlated the presence of this with good cognitive performance [20, 44]. Another showed no association using the Montreal Cognitive assessment, and cognitive performance and surrogate markers of CVD [21].
NIHSS scores, a marker of stroke severity, were reported infrequently. Most studies showed unadjusted scores of minor/moderate severity (NIHSS < 14). Scores were generally lower than those reported in HIV-negative control groups, indicating lesser stroke severity in PLWH. Our study from Malawi showed a median score of 12 in PLWH and 13.5 in HIV-negative cases [31]. Likewise, Kroon et al. showed a mean of 11 in PLWH and 14 in those HIV-negative individuals with stroke [32].
Discussion
Our systematic review found that the CVD landscape in PLWH residing in LMICs between 1990 and 2021 has evolved. Due to the success of ART uptake, PLWH are living longer. We found that among those manifesting with CVD, the median age has increased, and a greater number of patients are on ART in more contemporary cohorts. In turn, the CD4 + T-cell count has also increased over time. Moreover, the burden of overlapping CVD risk factors, notably hypertension, also increased. Mortality rates across the decades remained high but appeared not to differ compared to the HIV-negative populations. The numbers of studies published annually increased over the last 5 years in keeping with reports of an increasing burden of CVD in PLWH [1].
The top ten countries with the highest prevalence of HIV, ranging from 10–27%, were found in sub-Saharan Africa (SSA). Haiti is the LMIC outside of SSA with the highest HIV prevalence (1.9%) and has the 24th highest HIV prevalence globally [45]. In this review, the representation of the publications emerging from LMICs was a fair reflection of where HIV is most burdensome as 69% of included studies were from SSA. However, absolute numbers of publications were low overall, highlighting the limited data on HIV-associated CVD from LMICs.
Only a handful of studies in the last 5-years estimated a stroke burden, and these ranged from 3 to 12 per 1000 person-years [24, 25, 46]. The under-representation of stroke burden assessments in LMICs, especially SSA, has been a historical problem but improving [8]. With an ageing population and an overlapping burden of CVD risk factors in PLWH, we would have expected an increased number of those with neurocognitive impairment as well, but this was rarely reported. The reason is likely multifactorial, including (1) inconsistent coding; in 2017, cerebrovascular disease was coded separately from cardiovascular diseases in the ICD-11 classification; prior to this change, it is likely to have been underestimated, (2) challenges with cross-culture bias in neuropsychology testing tools, (3) an underrepresentation of neuroscience researchers in LMICs, who would typically undertake these studies [47,48,49]. A degree of education is often assumed with the tools needed to assess neurocognition and thus risks overestimating the burden, but this is not the current problem. Rather, a near complete absence of data on CVD contributions to neurocognitive impairment among PLWH residing in LMICs was noted. These limitations extend beyond HIV infection to data on the burden of dementia in LMICs and need addressing [50].
Notably, most studies had brain imaging to define a stroke and determine the stroke type. Ischaemic stroke was the predominant type of stroke, accounting for a prevalence > 85%; this is consistent with studies from HICs [4]. However, in approximately 40% of studies focused on aetiology, there was a preferential selection for ischaemic stroke type, underrepresenting HIV-associated intracerebral haemorrhagic stroke and limiting our ability to corroborate findings of an associated risk as described in HICs [4]. Beyond brain imaging, there was variability in the panel used to investigate CVD and define the aetiology. For example, treatable aetiologies such as opportunistic infections and cardioembolism, determined by CSF analysis and electrocardiogram/cardiac echocardiogram, respectively, were only performed in 60% of studies. A consensus approach to identifying stroke aetiology among PLWH, led by some of the authors, was published in 2017, where a minimum battery of tests was proposed to define the common aetiologies found in PLWH and presenting with CVD [39]. However, resource limitations result in difficulty obtaining even this limited battery of investigations in many LMIC settings, as does human resource limitations as neurologists and other stroke experts are often lacking in these settings.
Unsurprisingly, the outcome of CVD among PLWH remains poor. Although poorer health systems may play a part, the failure to systematically screen and manage treatable aetiologies may also be relevant. Additionally, the reporting of outcome measures was variable in terms of measures used and the timing of when events were measured. Guidance on standard reporting of outcome measures in stroke has been proposed and could be applied to CVD studies in PLWH [51, 52].
The striking rise in hypertension between 1990 and 2021 was apparent in our review. Already, policy implementation exists in how to reduce the burden of hypertension in PLWH (primary prevention), primarily by exploiting well-developed HIV health care systems [53]. However, the challenge with polypharmacy and drug interactions poses a different barrier in LMICs. Particular attention should be paid to secondary prevention as these individuals with an accrued disability may encounter additional barriers to accessing health systems and inadvertently be neglected.
A major limitation to this review is the dependence on mostly low-level observational studies, which limited our ability to pool data in a meta-analysis, and was subject to bias and confounding. Moreover, almost all studies were hospital-based, restricting our understanding of milder CVD cases at risk of subsequent events and further disability or fatal events in the community. Although three studies demonstrated excess CVD risk in PLWH, which is consistent with reports in HICs, we still have less understanding of CVD-associated cognitive risk in PLWH. Current data suggest that some of the underlying pathobiology of stroke and vascular-associated cognitive impairment might be interrelated [54]. Therefore, it is essential to develop a robust understanding of any vascular component involved in cognitive impairment in PLWH so that successful primary prevention strategies for stroke can be integrated with those for cognitive impairment. Investment in surveillance cohorts of PLWH across SSA focusing on non-communicable diseases is emerging, but more are needed. In time, this will give robust incidence, prevalence, mortality and disability metrics and inform policy [53, 55, 56]. However, standardised reporting of risk factors, aetiology and outcome will be crucial in supporting the advancement of this field.
Conclusions
The clinical phenotype of CVD among PLWH over the last three decades in LMICs has evolved and transitioned to an older group with overlapping cerebrovascular risk factors. There is an important need for further rigorous population-based studies and large observational cohort studies of PLWH in LMICs and to standardise reporting to facilitate understanding, guide appropriate interventions and evaluate its impact.
References
Papers of particular interest, published recently, have been highlighted as: •• Of major importance
Shah ASV, Stelzle D, Lee KK, et al. Global burden of atherosclerotic cardiovascular disease in people living with HIV: systematic review and meta-analysis. Circulation. 2018;138(11):1100–12.
Bhatta DN, Adhikari R, Karki S, Koirala AK, Wasti SP. Life expectancy and disparities in survival among HIV-infected people receiving antiretroviral therapy: an observational cohort study in Kathmandu, Nepal. BMJ Glob Health. 2019;4(3):e001319.
Pelchen-Matthews A, Ryom L, Borges AH, et al. Aging and the evolution of comorbidities among HIV-positive individuals in a European cohort. AIDS. 2018;32(16):2405–16.
Feinstein MJ, Hsue PY, Benjamin LA, et al. Characteristics, prevention, and management of cardiovascular disease in people living with HIV: a scientific statement from the American Heart Association. Circulation. 2019;140(2):e98–124. The first scientific statement on HIV and cardiovascular in PLWH.
Cruse B, Cysique LA, Markus R, Brew BJ. Cerebrovascular disease in HIV-infected individuals in the era of highly active antiretroviral therapy. J Neurovirol. 2012;18(4):264–76.
Chow FC, Regan S, Feske S, Meigs JB, Grinspoon SK, Triant VA. Comparison of ischemic stroke incidence in HIV-infected and non-HIV-infected patients in a US health care system. J Acquir Immune Defic Syndr. 2012;60(4):351–8.
Benjamin LA, Corbett EL, Connor MD, et al. HIV, antiretroviral treatment, hypertension, and stroke in Malawian adults: A case-control study. Neurology. 2016;86(4):324–33. This case-control study shows an association with HIV and stroke and shows the greatest risk is amongst those starting ART and immunosuppressed.
Akinyemi RO, Ovbiagele B, Adeniji OA, et al. Stroke in Africa: profile, progress, prospects and priorities. Nat Rev Neurol. 2021;17(10):634–56.
Benjamin LA, Bryer A, Emsley HC, Khoo S, Solomon T, Connor MD. HIV infection and stroke: current perspectives and future directions. Lancet Neurol. 2012;11(10):878–90.
Iloeje UH, Yuan Y, L’Italien G, et al. Protease inhibitor exposure and increased risk of cardiovascular disease in HIV-infected patients. HIV Med. 2005;6(1):37–44.
Fettig J, Swaminathan M, Murrill CS, Kaplan JE. Global epidemiology of HIV. Infect Dis Clin North Am. 2014;28(3):323–37.
Kharsany AB, Karim QA. HIV Infection and AIDS in Sub-Saharan Africa: current status, challenges and opportunities. Open AIDS J. 2016;10:34–48.
Mills KT, Stefanescu A, He J. The global epidemiology of hypertension. Nat Rev Nephrol. 2020;16(4):223–37.
Hyle EP, Mayosi BM, Middelkoop K, et al. The association between HIV and atherosclerotic cardiovascular disease in sub-Saharan Africa: a systematic review. BMC Public Health. 2017;17(1):954.
Abdallah A, Chang JL, O’Carroll CB, et al. Stroke in human immunodeficiency virus-infected individuals in sub-Saharan Africa (SSA): a systematic review. J Stroke Cerebrovasc Dis. 2018;27(7):1828–36.
Mochan A, Modi M, Modi G. Stroke in black South African HIV-positive patients: a prospective analysis. Stroke. 2003;34(1):10–5. First detailed description of the aetiology of stroke in PLWH.
Robbs JV, Paruk N. Management of HIV vasculopathy - a South African experience. Eur J Vasc Endovasc Surg. 2010;39 Suppl 1:S25-31.
Otedo AE, Oyoo GO, Obondi JO, Otieno CF. Vasculitis in HIV: report of eight cases. East Afr Med J. 2005;82(12):656–9.
Chow FC, Boscardin WJ, Mills C, et al. Cerebral vasoreactivity is impaired in treated, virally suppressed HIV-infected individuals. AIDS. 2016;30(1):45–55.
Hoffmann M. Stroke in the young in South Africa–an analysis of 320 patients. S Afr Med J. 2000;90(12):1226–37. This is one of the largest prospective studies of stroke in young adults, with specific attention given to aetiology.
Hiransuthikul A, Chutinet A, Sakulrak S, et al. Short communication: carotid intima-media thickness is not associated with neurocognitive impairment among people older than 50 years with and without HIV infection from Thailand. AIDS Res Hum Retroviruses. 2019;35(11–12):1170–3.
Sarfo FS, Opare-Sem O, Agyei M, et al. Risk factors for stroke occurrence in a low HIV endemic West African country: A case-control study. J Neurol Sci. 2018;395:8–16.
Sarfo FS, Nichols M, Agyei B, et al. Burden of subclinical carotid atherosclerosis and vascular risk factors among people living with HIV in Ghana. J Neurol Sci. 2019;397:103–11.
Mapoure YN, Nkongni IN, Luma HN, et al. Incidence of strokes in HIV-positive patients treated with long term antiretroviral treatment. Pan Afr Med J. 2016;24:45.
Lin HL, Muo CH, Lin CY, Chen HJ, Chen PC. Incidence of stroke in patients with HIV infection: a population-based study in Taiwan. PLoS One. 2019;14(5):e0217147.
Brites C, Nogueira RS, Gosuen GC, et al. Short communication: getting older with HIV: increasing frequency of comorbidities and polypharmacy in Brazilian HIV patients. AIDS Res Hum Retroviruses. 2019;35(11–12):1103–5.
Mochan A, Modi M, Modi G. Protein S deficiency in HIV associated ischaemic stroke: an epiphenomenon of HIV infection. J Neurol Neurosurg Psychiatry. 2005;76(10):1455–6.
Deshpande AK, Patnaik MM. Nonopportunistic neurologic manifestations of the human immunodeficiency virus: an Indian study. MedGenMed. 2005;7(4):2.
Tipping B, de Villiers L, Wainwright H, Candy S, Bryer A. Stroke in patients with human immunodeficiency virus infection. J Neurol Neurosurg Psychiatry. 2007;78(12):1320–4. One of the first studies to characterise HIV-associated vasculopathy.
Kumwenda JJ, Mateyu G, Kampondeni S, van Dam AP, van Lieshout L, Zijlstra EE. Differential diagnosis of stroke in a setting of high HIV prevalence in Blantyre, Malawi. Stroke. 2005;36(5):960–4.
Kamtchum-Tatuene J, Mwandumba H, Al-Bayati Z, et al. HIV is associated with endothelial activation despite ART, in a sub-Saharan African setting. Neurol Neuroimmunol Neuroinflamm. 2019;6(2):e531.
Kroon L, van Zyl DG, Schutte CM, Smit C, Hiesgen J. Risk factors for stroke in HIV-positive and-negative patients in Pretoria, South Africa. J Stroke Cerebrovasc Dis. 2021;30(8):105929.
Ellis JP, Kalata N, Joekes EC, et al. Ischemic stroke as a complication of cryptococcal meningitis and immune reconstitution inflammatory syndrome: a case report. BMC Infect Dis. 2018;18(1):520.
Ounjaijean S, Kulprachakarn K, Aurpibul L, et al. Cardiovascular risks in Asian HIV-infected patients receiving boosted-protease inhibitor-based antiretroviral treatment. J Infect Dev Ctries. 2021;15(2):289–96.
Juma K, Nyabera R, Mbugua S, et al. Cardiovascular risk factors among people living with HIV in rural Kenya: a clinic-based study. Cardiovasc J Afr. 2019;30(1):52–6.
Heikinheimo T, Chimbayo D, Kumwenda JJ, Kampondeni S, Allain TJ. Stroke outcomes in Malawi, a country with high prevalence of HIV: a prospective follow-up study. PLoS One. 2012;7(3):e33765. Describes stroke outcome in PLWH and compares to HIV negative populations.
Zimba S, Nutakki A, Chishimba L, et al. Risk factors and outcomes of HIV-associated stroke in Zambia. AIDS. 2021;35(13):2149–55. A comprehensive description of risk factors and outcome of stroke in PLWH.
Kuate LM, Tchuisseu LAK, Jingi AM, et al. Cardiovascular risk and stroke mortality in persons living with HIV: a longitudinal study in a hospital in Yaounde. Pan Afr Med J. 2021;40:8. Shows high mortality rates in PLWH in Cameroon following a stroke.
Benjamin LA, Bryer A, Lucas S, et al. Arterial ischemic stroke in HIV: defining and classifying etiology for research studies. Neurol Neuroimmunol Neuroinflamm. 2016;3(4):e254.
Benjamin LA, Allain TJ, Mzinganjira H, et al. The role of human immunodeficiency virus-associated vasculopathy in the etiology of stroke. J Infect Dis. 2017;216(5):545–53. Describes immune reconstitution vasculopathy in a HIV patient with advanced disease.
Lee B, Anekthananon T, Poungvarin N, Nilanont Y. Etiology and risk factors of stroke in HIV-infected patients in Siriraj Hospital: a case-control study. J Med Assoc Thai. 2012;95 Suppl 2:S227-234.
Wu L, Xiao J, Song Y, Gao G, Zhao H. The clinical characteristics and outcome of cryptococcal meningitis with AIDS in a tertiary hospital in China: an observational cohort study. BMC Infect Dis. 2020;20(1):912.
Yen YF, Jen I, Chen M, et al. Association of cytomegalovirus end-organ disease with stroke in people living with HIV/AIDS: a nationwide population-based cohort study. PLoS One. 2016;11(3):e0151684.
Chow FC, Wang H, Li Y, et al. Cerebral vasoreactivity evaluated by the breath-holding challenge correlates with performance on a cognitive screening test in persons living with treated HIV infection in China. J Acquir Immune Defic Syndr. 2018;79(3):e101–4.
Guide to Country Comparisons - The World Factbook". Retrieved 2021-01-17 from, www.cia.gov.
Sarfo FS, Norman B, Appiah L, Ovbiagele B. Factors associated with incidence of stroke and heart failure among people living with HIV in Ghana: Evaluating Vascular Event Risk while on Long-Term Antiretroviral Suppressive Therapy (EVERLAST) study. J Clin Hypertens (Greenwich). 2021;23(6):1252–9.
Shakir R, Norrving B. Stroke in ICD-11: the end of a long exile. Lancet. 2017;389(10087):2373.
Fernández AL, Abe J. Bias in cross-cultural neuropsychological testing: problems and possible solutions. Cult Brain. 2018;2018(6):1–38.
Cottler LB, Zunt J, Weiss B, Kamal AK, Vaddiparti K. Building global capacity for brain and nervous system disorders research. Nature. 2015;527(7578):S207-213.
Chibanda D, Benjamin L, Weiss HA, Abas M. Mental, neurological, and substance use disorders in people living with HIV/AIDS in low- and middle-income countries. J Acquir Immune Defic Syndr. 2014;67 Suppl 1:S54-67.
Reeves M, Lisabeth L, Williams L, et al. Patient-reported outcome measures (PROMs) for acute stroke: rationale, methods and future directions. Stroke. 2018;49(6):1549–56.
Salinas J, Sprinkhuizen SM, Ackerson T, et al. An international standard set of patient-centered outcome measures after stroke. Stroke. 2016;47(1):180–6.
Wong EB, Olivier S, Gunda R, et al. Convergence of infectious and non-communicable disease epidemics in rural South Africa: a cross-sectional, population-based multimorbidity study. Lancet Glob Health. 2021;9(7):e967–76.
Kalaria RN. Cerebrovascular disease and mechanisms of cognitive impairment: evidence from clinicopathological studies in humans. Stroke. 2012;43(9):2526–34.
Kabudula CW, Houle B, Collinson MA, et al. Socioeconomic differences in mortality in the antiretroviral therapy era in Agincourt, rural South Africa, 2001–13: a population surveillance analysis. Lancet Glob Health. 2017;5(9):e924–35.
Peterson I, Ntsui N, Jambo K, et al. Evaluating the reactivation of herpesviruses and inflammation as cardiovascular and cerebrovascular risk factors in antiretroviral therapy initiators in an African HIV-infected population (RHICCA): a protocol for a longitudinal cohort study. BMJ Open. 2019;9(9):e025576.
Perriëns JH, Mussa M, Luabeya MK, et al. Neurological complications of HIV-1-seropositive internal medicine inpatients in Kinshasa, Zaire. J Acquir Immune Defic Syndr. 1992;5(4):333–40.
Hoffmann M, Berger JR, Nath A, Rayens M. Cerebrovascular disease in young, HIV-infected, black Africans in the KwaZulu Natal province of South Africa. J Neurovirol. 2000;6(3):229–36.
Connor MD, Thorogood M, Casserly B, Dobson C, Warlow CP. Prevalence of stroke survivors in rural South Africa: results from the Southern Africa Stroke Prevention Initiative (SASPI) Agincourt field site. Stroke. 2004;35(3):627–32.
Patel VB, Sacoor Z, Francis P, Bill PL, Bhigjee AI, Connolly C. Ischemic stroke in young HIV-positive patients in Kwazulu-Natal, South Africa. Neurology. 2005;65(5):759–61.
Joshi R, Cardona M, Iyengar S, et al. Chronic diseases now a leading cause of death in rural India–mortality data from the Andhra Pradesh Rural Health Initiative. Int J Epidemiol. 2006;35(6):1522–9.
Jowi JO, Mativo PM, Musoke SS. Clinical and laboratory characteristics of hospitalised patients with neurological manifestations of HIV/AIDS at the Nairobi hospital. East Afr Med J. 2007;84(2):67–76.
Jowi JO, Mativo PM. Pathological sub-types, risk factors and outcome of stroke at the Nairobi Hospital, Kenya. East Afr Med J. 2008;85(12):572–81.
Andrade AC, Ladeia AM, Netto EM, et al. Cross-sectional study of endothelial function in HIV-infected patients in Brazil. AIDS Res Hum Retroviruses. 2008;24(1):27–33.
Onwuchekwa AC, Asekomeh EG, Iyagba AM, Onung SI. Medical mortality in the Accident and Emergency Unit of the University of Port Harcourt Teaching Hospital. Niger J Med. 2008;17(2):182–5.
Silva EF, Bassichetto KC, Lewi DS. Lipid profile, cardiovascular risk factors and metabolic syndrome in a group of AIDS patients. Arq Bras Cardiol. 2009;93(2):113–8.
Maduagwu SM, Ezeukwu AO, Saidu IA, Sangodeyi BJ, Jaiyeola OA. Co-morbidities and socio-demographic distribution of stroke survivors referred for physiotherapy in a Nigerian Teaching Hospital: a retrospective study. Niger Postgrad Med J. 2012;19(4):240–3.
Maier D, Doppler M, Gasser A, et al. Imaging-based disease pattern in a consecutive series of cranial CTs and MRIs in a rural and an urban Tanzanian hospital: a comparative, retrospective, neuroradiological analysis. Wien Klin Wochenschr. 2010;122 Suppl 3:40–6.
Fourie C, van Rooyen J, Pieters M, Conradie K, Hoekstra T, Schutte A. Is HIV-1 infection associated with endothelial dysfunction in a population of African ancestry in South Africa? Cardiovasc J Afr. 2011;22(3):134–40.
Longo-Mbenza B, Mashi ML, Tshikwela ML, Mokondjimobe E, Gombet T, Ellenga-Mbolla B, Okwe AN, Kabangu NK, Fuele SM. Relationship between younger age, autoimmunity, cardio-metabolic risk, oxidative stress, HAART, and ischemic stroke in Africans with HIV/AIDS. Int Sch Res Not. 2011;2011:897908
Neto JP, Lyra IM, Reis MG, Goncalves MS. The association of infection and clinical severity in sickle cell anaemia patients. Trans R Soc Trop Med Hyg. 2011;105(3):121–6.
Falcão Mda C, Zírpoli JC, Albuquerque VM, et al. Association of biomarkers with atherosclerosis and risk for coronary artery disease in patients with HIV. Arq Bras Cardiol. 2012;99(5):971–8.
Owolabi LF, Ibrahim A. Stroke in young adults: a prospective study from northwestern Nigeria. Int Sch Res Not. 2012;2012:468706
Ngatchou W, Lemogoum D, Ndobo P, et al. Increased burden and severity of metabolic syndrome and arterial stiffness in treatment-naïve HIV+ patients from Cameroon. Vasc Health Risk Manag. 2013;9:509–16.
Ssinabulya I, Kayima J, Longenecker C, et al. Subclinical atherosclerosis among HIV-infected adults attending HIV/AIDS care at two large ambulatory HIV clinics in Uganda. PLoS ONE. 2014;9(2):e89537.
Mossong J, Byass P, Herbst K. Who died of what in rural KwaZulu-Natal, South Africa: a cause of death analysis using InterVA-4. Glob Health Action. 2014;7:25496.
Smit M, Olney J, Ford NP, et al. The growing burden of noncommunicable disease among persons living with HIV in Zimbabwe. AIDS. 2018;32(6):773–82.
Nakibuuka J, Sajatovic M, Nankabirwa J, et al. Stroke-risk factors differ between rural and urban communities: population survey in central Uganda. Neuroepidemiology. 2015;44(3):156–65.
Schoffelen AF, de Groot E, Tempelman HA, Visseren FL, Hoepelman AI, Barth RE. Carotid intima media thickness in mainly female HIV-infected subjects in rural south africa: association with cardiovascular but not HIV-related factors. Clin Infect Dis. 2015;61(10):1606–14.
Pacheco AG, Grinsztejn B, da Fonseca MJ, et al. Traditional risk factors are more relevant than HIV-specific ones for carotid intima-media thickness (cIMT) in a Brazilian cohort of HIV-infected patients. PLoS ONE. 2015;10(2):e0117461.
Asiki G, Stockdale L, Kasamba I, et al. Pilot study of antibodies against varicella zoster virus and human immunodeficiency virus in relation to the risk of developing stroke, nested within a rural cohort in Uganda. Trop Med Int Health. 2015;20(10):1306–10.
Zimba S, Ntanda PM, Lakhi S, Atadzhanov M. HIV infection, hypercoagulability and ischaemic stroke in adults at the University Teaching Hospital in Zambia: a case control study. BMC Infect Dis. 2017;17(1):354.
Fourie CM, Schutte AE, Smith W, Kruger A, van Rooyen JM. Endothelial activation and cardiometabolic profiles of treated and never-treated HIV infected Africans. Atherosclerosis. 2015;240(1):154–60.
Valenzuela-Rodríguez G, Mezones-Holguín E, Mendo-Urbina F, Rodríguez-Morales AJ. Cardiovascular disease in human immunodeficiency virus-infection as a cause of hospitalization: a case-series in a General Hospital in Peru. Braz J Infect Dis. 2015;19(4):431–5.
Heikinheimo T, Chimbayo D. Quality of life after first-ever stroke: an interview-based study from Blantyre, Malawi. Malawi Med J. 2015;27(2):50–4.
Pacheco AG, Grinsztejn B, Fonseca Mde J, et al. HIV infection is not associated with carotid intima-media thickness in Brazil: a cross-sectional analysis from the INI/ELSA-Brasil study. PLoS ONE. 2016;11(7):e0158999.
Okeng’o K, Chillo P, Gray WK, Walker RW, Matuja W. Early mortality and associated factors among patients with stroke admitted to a large teaching hospital in Tanzania. J Stroke Cerebrovasc Dis. 2017;26(4):871–8.
Divala OH, Amberbir A, Ismail Z, et al. The burden of hypertension, diabetes mellitus, and cardiovascular risk factors among adult Malawians in HIV care: consequences for integrated services. BMC Public Health. 2016;16(1):1243.
Osegbe ID, Soriyan OO, Ogbenna AA, Okpara HC, Azinge EC. Risk factors and assessment for cardiovascular disease among HIV-positive patients attending a Nigerian tertiary hospital. Pan Afr Med J. 2016;23:206.
Rodriguez-Fernandez R, Ng N, Susilo D, Prawira J, Bangs MJ, Amiya RM. The double burden of disease among mining workers in Papua, Indonesia: at the crossroads between Old and New health paradigms. BMC Public Health. 2016;16(1):951.
Gleason RL Jr, Caulk AW, Seifu D, et al. Efavirenz and ritonavir-boosted lopinavir use exhibited elevated markers of atherosclerosis across age groups in people living with HIV in Ethiopia. J Biomech. 2016;49(13):2584–92.
Sarfo FS, Awuah DO, Nkyi C, Akassi J, Opare-Sem OK, Ovbiagele B. Recent patterns and predictors of neurological mortality among hospitalized patients in Central Ghana. J Neurol Sci. 2016;363:217–24.
Siedner MJ, Kim JH, Nakku RS, et al. Persistent immune activation and carotid atherosclerosis in HIV-infected Ugandans receiving antiretroviral therapy. J Infect Dis. 2016;213(3):370–8.
Cumming K, Tiamkao S, Kongbunkiat K, et al. Impact of HIV on inpatient mortality and complications in stroke in Thailand: a National Database Study. Epidemiol Infect. 2017;145(6):1285–91.
Mosepele M, Hemphill LC, Moloi W, et al. Pre-clinical carotid atherosclerosis and sCD163 among virally suppressed HIV patients in Botswana compared with uninfected controls. PLoS ONE. 2017;12(6):e0179994.
Mosepele M, Hemphill LC, Palai T, et al. Cardiovascular disease risk prediction by the American College of Cardiology (ACC)/American Heart Association (AHA) Atherosclerotic Cardiovascular Disease (ASCVD) risk score among HIV-infected patients in sub-Saharan Africa. PLoS One. 2017;12(2):e0172897.
Siwamogsatham S, Chutinet A, Vongsayan P, et al. Low CD4 cell counts are associated with carotid plaque and intima-media thickness in virologically suppressed HIV-infected asians older than 50 years. AIDS Res Hum Retroviruses. 2019;35(11–12):1160–9.
Sharma SR, Hussain M, Habung H. Neurological manifestations of HIV-AIDS at a tertiary care institute in North Eastern India. Neurol India. 2017;65(1):64–8.
Feinstein MJ, Kim JH, Bibangambah P, et al. Ideal cardiovascular health and carotid atherosclerosis in a mixed cohort of HIV-infected and uninfected Ugandans. AIDS Res Hum Retroviruses. 2017;33(1):49–56.
Kaseke F, Stewart A, Gwanzura L, Hakim J, Chikwasha V. Clinical characteristics and outcomes of patients with stroke admitted to three tertiary hospitals in Zimbabwe: a retrospective one-year study. Malawi Med J. 2017;29(2):177–82.
Salmazo PS, Bazan SGZ, Shiraishi FG, Bazan R, Okoshi K, Hueb JC. Frequency of subclinical atherosclerosis in Brazilian HIV-infected patients. Arq Bras Cardiol. 2018;110(5):402–10.
Ekrikpo UE, Akpan EE, Ekott JU, Bello AK, Okpechi IG, Kengne AP. Prevalence and correlates of traditional risk factors for cardiovascular disease in a Nigerian ART-naive HIV population: a cross-sectional study. BMJ Open. 2018;8(7):e019664.
Nonterah EA, Boua PR, Klipstein-Grobusch K, et al. Classical cardiovascular risk factors and HIV are associated with carotid intima-media thickness in adults from sub-Saharan Africa: findings from H3Africa AWI-Gen study. J Am Heart Assoc. 2019;8(14): e011506.
Lai YJ, Chen YY, Huang HH, Ko MC, Chen CC, Yen YF. Incidence of cardiovascular diseases in a nationwide HIV/AIDS patient cohort in Taiwan from 2000 to 2014. Epidemiol Infect. 2018;146(16):2066–71.
Bergmann T, Sengupta S, Bhrushundi MP, Kulkarni H, Sengupta PP, Fergus I. HIV related stigma, perceived social support and risk of premature atherosclerosis in South Asians. Indian Heart J. 2018;70(5):630–6.
Kamtchum-Tatuene J, Al-Bayati Z, Mwandumba HC, Solomon T, Christmas SE, Benjamin LA. Serum concentration of anti-Cytomegalovirus IgG and ischaemic stroke in patients with advanced HIV infection in Malawi. PLoS One. 2018;13(11):e0208040.
Kiragga AN, Mubiru F, Kambugu AD, Kamya MR, Castelnuovo B. A decade of antiretroviral therapy in Uganda: what are the emerging causes of death? BMC Infect Dis. 2019;19(1):77.
Aurpibul L, Srithanaviboonchai K, Rerkasem K, Tangmunkongvorakul A, Sitthi W, Musumari PM. Prevalence of Subclinical atherosclerosis and risk of atherosclerotic cardiovascular disease in older adults living with HIV. AIDS Res Hum Retroviruses. 2019;35(11–12):1136–42.
Mapoure Njankouo Y, Mondomobe Atchom C, Halle MP, Mbatchou Ngahane BH, Luma NH. Prevalence of HIV infection among stroke patients in Douala. Med Sante Trop. 2019;29(2):184–9.
Yang IT, Hemphill LC, Kim JH, et al. To fast or not to fast: Lipid measurement and cardiovascular disease risk estimation in rural sub-Saharan Africa. J Glob Health. 2020;10(1):010407.
Belisário AR, Blatyta PF, Vivanco D, et al. Association of HIV infection with clinical and laboratory characteristics of sickle cell disease. BMC Infect Dis. 2020;20(1):638.
Matuja SS, Munseri P, Khanbhai K. The burden and outcomes of stroke in young adults at a tertiary hospital in Tanzania: a comparison with older adults. BMC Neurol. 2020;20(1):206.
Vos AG, Dodd CN, Delemarre EM, et al. Patterns of immune activation in HIV and non HIV subjects and its relation to cardiovascular disease risk. Front Immunol. 2021;12:647805.
Siedner MJ, Bibangambah P, Kim JH, et al. Treated HIV infection and progression of carotid atherosclerosis in rural Uganda: a prospective observational cohort study. J Am Heart Assoc. 2021;10(12):e019994.
Osaigbovo GO, Amusa GA, Salaam AJ, et al. Predictors and prognosis of stroke in Jos, North-Central Nigeria. West Afr J Med. 2021;38(5):478–85.
Hiransuthikul A, Chutinet A, Suwanwela NC. Short communication: ischemic stroke subtypes among Thai HIV-infected patients: a 12-year retrospective study. AIDS Res Hum Retroviruses. 2021;37(9):627–30.
Dirajlal-Fargo S, Sattar A, Yu J, et al. Lipidome association with vascular disease and inflammation in HIV+ Ugandan children. AIDS. 2021;35(10):1615–23.
Nutakki A, Chomba M, Chishimba L, et al. Risk factors and outcomes of hospitalized stroke patients in Lusaka, Zambia. J Neurol Sci. 2021;424:117404.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Central Nervous System and Cognition
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ransley, G., Zimba, S., Gadama, Y. et al. Trends and Clinical Characteristics of HIV and Cerebrovascular Disease in Low- and Middle-Income Countries (LMICs) Between 1990 and 2021. Curr HIV/AIDS Rep 19, 548–565 (2022). https://doi.org/10.1007/s11904-022-00627-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11904-022-00627-9