Abstract
Purpose of Review
Hepatic encephalopathy (HE) is a complication of liver dysfunction and portosystemic shunting. The purpose of this review is to discuss the management of HE.
Recent Findings
Traditional therapies include lactulose and rifaximin, but other treatment options have recently emerged. Branched chain amino acids are involved in the Krebs cycle and have an inverse relationship with ammonia. Flumazenil, a benzodiazepine antagonist, has a negative effect on the gamma-aminobutyric acid receptor, which plays a role in cognitive deficits. Because of microbiome changes, fecal microbiota transplantation and probiotics are emerging therapeutic considerations.
Summary
Despite multiple therapies for HE, the emerging options have shown varying degrees of evidence. Future studies are needed to examine the impact of each therapy on the different grades of HE, as most studies showed benefit for primarily lower grades. Future studies should also investigate whether certain therapeutic combinations are favored.

Similar content being viewed by others
References
Vilstrup H, Amodio P, Bajaj J, Cordoba J, Ferenci P, Mullen KD, et al. Hepatic encephalopathy in chronic liver disease: 2014 Practice Guideline by the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver. Hepatology. 2014;60(2):715–35. https://doi.org/10.1002/hep.27210.
Rose CF, Amodio P, Bajaj JS, Dhiman RK, Montagnese S, Taylor-Robinson SD, et al. Hepatic encephalopathy: novel insights into classification, pathophysiology and therapy. J Hepatol. 2020;73(6):1526–47. https://doi.org/10.1016/j.jhep.2020.07.013.
European Association for the Study of the Liver. Electronic address: easloffice@easloffice.eu; European Association for the Study of the Liver. EASL Clinical Practice Guidelines on the management of hepatic encephalopathy. J Hepatol. 2022;77(3):807–824. https://doi.org/10.1016/j.jhep.2022.06.001. Erratum in: J Hepatol. 2023;79(5):1340.
Weissenborn K. Hepatic encephalopathy: definition, clinical grading and diagnostic principles. Drugs. 2019;79(Suppl 1):5–9. https://doi.org/10.1007/s40265-018-1018-z.
Romero-Gómez M, et al. Hepatic encephalopathy in patients with acute decompensation of cirrhosis and acute-on-chronic liver failure. J Hepatol. 2015;62(2):437–47. https://doi.org/10.1016/j.jhep.2014.09.005.
Aldridge DR, et al. Pathogenesis of hepatic encephalopathy: role of ammonia and systemic inflammation. J Clin Exp Hepatol. 2015;1:S7–20. https://doi.org/10.1016/j.jceh.2014.06.004.
Romero-Gómez M. Role of phosphate-activated glutaminase in the pathogenesis of hepatic encephalopathy. Metab Brain Dis. 2005;20(4):319–25. https://doi.org/10.1007/s11011-005-7913-5.
Jalan R, Fernandez J, Wiest R, Schnabl B, Moreau R, Angeli P, et al. Bacterial infections in cirrhosis: a position statement based on the EASL Special Conference 2013. J Hepatol. 2014;60(6):1310–24. https://doi.org/10.1016/j.jhep.2014.01.024.
Rose C, Butterworth RF, Zayed J, Normandin L, Todd K, Michalak A, et al. Manganese deposition in basal ganglia structures results from both portal-systemic shunting and liver dysfunction. Gastroenterology. 1999;117(3):640–4. https://doi.org/10.1016/s0016-5085(99)70457-9.
Rovira A, et al. MR imaging findings in hepatic encephalopathy. AJNR Am J Neuroradiol. 2008;9:1612–21. https://doi.org/10.3174/ajnr.A1139.
Poo JL, Rosas-Romero R, Rodríguez F, Silencio JL, Muñoz R, Bourges H, et al. Serum zinc concentrations in two cohorts of 153 healthy subjects and 100 cirrhotic patients from Mexico City. Dig Dis. 1995;2:136–42. https://doi.org/10.1159/000171495.
Takuma Y, et al. Clinical trial: oral zinc in hepatic encephalopathy. Aliment Pharmacol Ther. 2010;32(9):1080–90. https://doi.org/10.1111/j.1365-2036.2010.04448.x.
Aceto P, et al. Postoperative cognitive dysfunction after liver transplantation. Gen Hosp Psychiatry. 2015;37(2):109–15. https://doi.org/10.1016/j.genhosppsych.2014.12.001.
Amodio P, Biancardi A, Montagnese S, Angeli P, Iannizzi P, Cillo U, et al. Neurological complications after orthotopic liver transplantation. Dig Liver Dis. 2007;39(8):740–7. https://doi.org/10.1016/j.dld.2007.05.004.
Schiano TD. Treatment options for hepatic encephalopathy. Pharmacotherapy. 2010;30(5 Pt 2):16S-21S. https://doi.org/10.1592/phco.30.pt2.16S.
FiatiKenston SS, et al. Mechanistic insight, diagnosis, and treatment of ammonia-induced hepatic encephalopathy. J Gastroenterol Hepatol. 2019;34(1):31–9. https://doi.org/10.1111/jgh.14408.
Ferenci P. Hepatic encephalopathy in adults: treatment. Post TW, ed. UpToDate. Waltham, MA: UpToDate Inc. http://www.uptodate.com. Accessed September 2, 2021.
Mohammad RA, et al. Combination therapy for the treatment and prevention of hepatic encephalopathy. Ann Pharmacother. 2012;46(11):1559–63. https://doi.org/10.1345/aph.1R146.
Ullah MI, Alameen AAM, Al-Oanzi ZH, Eltayeb LB, Atif M, Munir MU, et al. Biological role of zinc in liver cirrhosis: an updated review. Biomedicines. 2023;11(4):1094. https://doi.org/10.3390/biomedicines11041094.
Grüngreiff K, et al. The role of zinc in liver cirrhosis. Ann Hepatol. 2016;15(1):7–16. https://doi.org/10.5604/16652681.1184191.
Yoshida Y, Higashi T, Nouso K, Nakatsukasa H, Nakamura SI, Watanabe A, et al. Effects of zinc deficiency/zinc supplementation on ammonia metabolism in patients with decompensated liver cirrhosis. Acta Med Okayama. 2001;55(6):349–55. https://doi.org/10.18926/AMO/32003.
Plum LM, et al. The essential toxin: impact of zinc on human health. Int J Environ Res Public Health. 2010;7(4):1342–65. https://doi.org/10.3390/ijerph7041342.
Sengupta S, Wroblewski K, Aronsohn A, Reau N, Reddy KG, Jensen D, et al. Screening for zinc deficiency in patients with cirrhosis: when should we start? Dig Dis Sci. 2015;60(10):3130–5. https://doi.org/10.1007/s10620-015-3613-0.
Deep V, Sondhi S, Gupta S. Assessment of serum zinc levels in patients with decompensated cirrhosis of the liver and its association with disease severity and hepatic encephalopathy: a prospective observational study from North India. Cureus. 2023;15(6):e41207. https://doi.org/10.7759/cureus.41207.
Janyajirawong R, et al. Efficacy of zinc supplement in minimal hepatic encephalopathy: a prospective, randomized controlled study (Zinc-MHE Trial). Asian Pac J Cancer Prev. 2021;22(9):2879–87. https://doi.org/10.31557/APJCP.2021.22.9.2879.
Shen YC, et al. Zinc supplementation in patients with cirrhosis and hepatic encephalopathy: a systematic review and meta-analysis. Nutr J. 2019;18(1):34. https://doi.org/10.1186/s12937-019-0461-3.
Diglio DC, et al. Role of zinc supplementation in the management of chronic liver diseases: a systematic review and meta-analysis. Ann Hepatol. 2020;19(2):190–6. https://doi.org/10.1016/j.aohep.2019.08.011.
Duncan A, et al. The risk of copper deficiency in patients prescribed zinc supplements. J Clin Pathol. 2015;68(9):723–5. https://doi.org/10.1136/jclinpath-2014-202837.
Institute of Medicine (US) Committee on nutrition, trauma, and the brain; Erdman J, Oria M, Pillsbury L, editors. Nutrition and traumatic brain injury: improving acute and subacute health outcomes in military personnel. Washington (DC): National Academies Press (US); 2011. 8, Branched-Chain Amino Acids. Available from: https://www.ncbi.nlm.nih.gov/books/NBK209312/
Holecek M. Branched-chain amino acids and ammonia metabolism in liver disease: therapeutic implications. Nutrition. 2013;29(10):1186–91. https://doi.org/10.1016/j.nut.2013.01.022.
Gluud LL, Dam G, Les I, Marchesini G, Borre M, Aagaard NK, et al. Branched-chain amino acids for people with hepatic encephalopathy. Cochrane Database Syst Rev. 2017;5:CD001939. https://doi.org/10.1002/14651858.CD001939.pub4.
Swansson WD, et al. Management of minimal and overt hepatic encephalopathy with branched-chain amino acids: a review of the evidence. Eur J Gastroenterol Hepatol. 2023;35(8):812–21. https://doi.org/10.1097/MEG.0000000000002595.
Amrein R, et al. Flumazenil in benzodiazepine antagonism. Actions and clinical use in intoxications and anaesthesiology. Med Toxicol Adverse Drug Exp. 1987;2(6):411–29. https://doi.org/10.1007/BF03259876.
Rajpurohit S et al 2022 Novel Drugs for the management of hepatic encephalopathy: still a long journey to travel. J Clin Exp Hepatol. 12(4):1200–1214 https://doi.org/10.1016/j.jceh.2022.01.012
Goh ET, et al. Flumazenil versus placebo or no intervention for people with cirrhosis and hepatic encephalopathy. Cochrane Database Syst Rev. 2017;8(8):CD002798. https://doi.org/10.1002/14651858.CD002798.pub4.
Laccetti M, et al. Flumazenil in the treatment of acute hepatic encephalopathy in cirrhotic patients: a double blind randomized placebo controlled study. Dig Liver Dis. 2000;32(4):335–8. https://doi.org/10.1016/s1590-8658(00)80027-4.
Chen Y, Yang F, Lu H, Wang B, Chen Y, Lei D, et al. Characterization of fecal microbial communities in patients with liver cirrhosis. Hepatology. 2011;54(2):562–72. https://doi.org/10.1002/hep.24423.
Bajaj JS, Heuman DM, Hylemon PB, Sanyal AJ, White MB, Monteith P, et al. Altered profile of human gut microbiome is associated with cirrhosis and its complications. J Hepatol. 2014;60(5):940–7. https://doi.org/10.1016/j.jhep.2013.12.019.
Bajaj JS, Kassam Z, Fagan A, Gavis EA, Liu E, Cox IJ, et al. Fecal microbiota transplant from a rational stool donor improves hepatic encephalopathy: a randomized clinical trial. Hepatology. 2017;66(6):1727–38. https://doi.org/10.1002/hep.29306.
Gedgaudas R, Bajaj JS, Skieceviciene J, Valantiene I, Kiudeliene E, Bang C, Franke A, Schreiber S, Kupcinskas J. Sterile fecal filtrate from a healthy donor improves microbial diversity in patients with hepatic encephalopathy. J Gastrointestin Liver Dis. 2023;32(3):332–338. https://doi.org/10.15403/jgld-4906.
Dhiman RK, Rana B, Agrawal S, Garg A, Chopra M, Thumburu KK, et al. Probiotic VSL#3 reduces liver disease severity and hospitalization in patients with cirrhosis: a randomized, controlled trial. Gastroenterology. 2014;147(6):1327-37.e3. https://doi.org/10.1053/j.gastro.2014.08.031.
Dalal R, et al. Probiotics for people with hepatic encephalopathy. Cochrane Database Syst Rev. 2017;2(2):008716. https://doi.org/10.1002/14651858.CD008716.pub3.
Rahimi RS, et al. Lactulose vs polyethylene glycol 3350–electrolyte solution for treatment of overt hepatic encephalopathy: the HELP randomized clinical trial. JAMA Intern Med. 2014;174(11):1727–33. https://doi.org/10.1001/jamainternmed.2014.4746.
Naderian M, et al. Polyethylene glycol and lactulose versus lactulose alone in the treatment of hepatic encephalopathy in patients with cirrhosis: a non-inferiority randomized controlled trial. Middle East J Dig Dis. 2017;9(1):12–9. https://doi.org/10.15171/mejdd.2016.46.
Shehata HH, Elfert AA, Abdin AA, Soliman SM, Elkhouly RA, Hawash NI, et al. Randomized controlled trial of polyethylene glycol versus lactulose for the treatment of overt hepatic encephalopathy. Eur J Gastroenterol Hepatol. 2018;30(12):1476–81. https://doi.org/10.1097/MEG.0000000000001267.
Is B, Bombassaro IZ, Tovo CV, de Mattos ÂZ, Ahlert M, Chiesa T, et al. Albumin in the management of hepatic encephalopathy: a systematic review and meta-analysis. Ann Hepatol. 2021;26:100541. https://doi.org/10.1016/j.aohep.2021.100541.
Sharma BC, Singh J, Srivastava S, Sangam A, Mantri AK, Trehanpati N, et al. Randomized controlled trial comparing lactulose plus albumin versus lactulose alone for treatment of hepatic encephalopathy. J Gastroenterol Hepatol. 2017;32(6):1234–9. https://doi.org/10.1111/jgh.13666.
Simón-Talero M, García-Martínez R, Torrens M, Augustin S, Gómez S, Pereira G, et al. Effects of intravenous albumin in patients with cirrhosis and episodic hepatic encephalopathy: a randomized double-blind study. J Hepatol. 2013;59(6):1184–92. https://doi.org/10.1016/j.jhep.2013.07.020.
Philips CA, et al. Portosystemic shunts and refractory hepatic encephalopathy: patient selection and current options. Hepat Med. 2019;25(11):23–34. https://doi.org/10.2147/HMER.S169024.
Laleman W, Simon-Talero M, Maleux G, Perez M, Ameloot K, Soriano G, et al. EASL-CLIF-Consortium. Embolization of large spontaneous portosystemic shunts for refractory hepatic encephalopathy: a multicenter survey on safety and efficacy. Hepatology. 2013;57(6):2448–57. https://doi.org/10.1002/hep.26314.
Goffaux A, et al. Improving the prognosis before and after liver transplantation: is muscle a game changer? World J Gastroenterol. 2022;28(40):5807–17. https://doi.org/10.3748/wjg.v28.i40.5807.
Nardelli S, Lattanzi B, Torrisi S, Greco F, Farcomeni A, Gioia S, et al. Sarcopenia is risk factor for development of hepatic encephalopathy after transjugular intrahepatic portosystemic shunt placement. Clin Gastroenterol Hepatol. 2017;15(6):934–6. https://doi.org/10.1016/j.cgh.2016.10.028.
Author information
Authors and Affiliations
Contributions
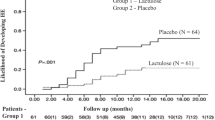
B.L.M. and D.M.H.C. designed the outline and reviewed and edited the manuscript and figure. M.C.L. and J.T.C. wrote the main manuscript text and prepared the figure.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Conflict of Interest
The authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, M.C., Chumbe, J.T., Chascsa, D.M.H. et al. Current Management of Hepatic Encephalopathy. Curr Hepatology Rep 23, 73–80 (2024). https://doi.org/10.1007/s11901-023-00627-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11901-023-00627-2