Abstract
Purpose of Review
The management of myelofibrosis is risk-adapted when considering transplant eligibility and symptom-directed, prioritizing the most burdensome symptoms for the patient. Unfortunately, myelofibrosis-anemia is common, multifactorial in its origin, and impactful regarding prognosis. While clinical trials are advised, not all patients have convenient access, and therefore, hematologists should be aware of the data supporting the use of conventional agents such as erythropoietin-stimulating agents, steroid treatments (danazol and prednisone), and immunomodulatory drugs (thalidomide and lenalidomide). This review summarizes the conventional approach to treating myelofibrosis-anemia and highlights recent data from 3 novel agents that are under phase 3 evaluation.
Recent Findings
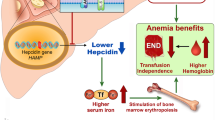
Momelotonib is a JAK1/2 and ACVR1 inhibitor that has demonstrated not only improvements in splenomegaly and symptoms, but also amelioration of anemia on the SIMPLIFY 1 and 2 clinical trial program. This may occur through suppression of hepcidin production. Luspatercept promotes late-stage hematopoiesis, and the phase 2 study has shown promise in ameliorating anemia as a monotherapy, and especially in combination with ruxolitinib. Finally, CP-0160, a BET inhibitor, has shown efficacy as an anemia-directed agent, when used as monotherapy and in combination. This agent reduces cytokine production and promotes erythroid differentiation. Results have been presented for patients previously treated with JAK inhibitors, as well as those who were naïve to JAK inhibitor therapy.
Summary
Safety and effectiveness are reviewed for both conventional and selected novel agents used in the treatment of MF-anemia. A practical approach to treatment is presented, and data from ASH 2020 are presented.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Dameshek W. Some speculations on the myeloproliferative syndromes. Blood. 1951;6(4):372–5.
Marneth AE, Mullally A. The molecular genetics of myeloproliferative neoplasms. Cold Spring Harb Perspect Med. 2020;10(2):a034876. https://doi.org/10.1101/cshperspect.a034876.
Verstovsek S, et al. Long-term treatment with ruxolitinib for patients with myelofibrosis: 5-year update from the randomized, double-blind, placebo-controlled, phase 3 COMFORT-I trial. J Hematol Oncol. 2017;10(1):55.
Harrison CN, et al. Long-term findings from COMFORT-II, a phase 3 study of ruxolitinib vs best available therapy for myelofibrosis. Leukemia. 2016;30(8):1701–7.
Vannucchi AM. Ruxolitinib versus standard therapy for the treatment of polycythemia vera. N Engl J Med. 2015;372(17):1670–1.
Pardanani A, et al. Safety and efficacy of fedratinib in patients with primary or secondary myelofibrosis: a randomized clinical trial. JAMA Oncol. 2015;1(5):643–51.
Harrison CN, et al. Janus kinase-2 inhibitor fedratinib in patients with myelofibrosis previously treated with ruxolitinib (JAKARTA-2): a single-arm, open-label, non-randomised, phase 2, multicentre study. Lancet Haematol. 2017;4(7):e317–24.
Guglielmelli P, et al. MIPSS70: mutation-enhanced international prognostic score system for transplantation-age patients with primary myelofibrosis. J Clin Oncol. 2018;36(4):310–8.
Myeloproliferative neoplasms. NCCN Guidelines 2021 [cited 2021 2/22/21].
Cervantes F. How I treat myelofibrosis. Blood. 2014;124(17):2635–42.
• Elli EM, et al. Deferasirox in the management of iron-overload in patients with myelofibrosis: a multicentre study from the Rete Ematologica Lombarda (IRON-M study). Br J Haematol. 2019;186(5):e123–6. The role of iron chelation is unclear in Myelofibrosis. This is a multicenter study showed that 43% had an erythroid response to iron chelation.
Hernandez-Boluda JC, et al. Predictive factors for anemia response to erythropoiesis-stimulating agents in myelofibrosis. Eur J Haematol. 2017;98(4):407–14.
Crisa E, et al. The use of erythropoiesis-stimulating agents is safe and effective in the management of anaemia in myelofibrosis patients treated with ruxolitinib. Br J Haematol. 2018;182(5):701–4.
Hernandez-Boluda JC, et al. Long-term results of prednisone treatment for the anemia of myelofibrosis. Leuk Lymphoma. 2016;57(1):120–4.
Cervantes F, et al. Danazol therapy for the anemia of myelofibrosis: assessment of efficacy with current criteria of response and long-term results. Ann Hematol. 2015;94(11):1791–6.
Gowin K, et al. Multicenter phase 2 study of combination therapy with ruxolitinib and danazol in patients with myelofibrosis. Leuk Res. 2017;60:31–5.
Luo X, et al. Thalidomide plus prednisone with or without danazol therapy in myelofibrosis: a retrospective analysis of incidence and durability of anemia response. Blood Cancer J. 2018;8(1):9.
Tefferi A, et al. A randomized study of pomalidomide vs placebo in persons with myeloproliferative neoplasm-associated myelofibrosis and RBC-transfusion dependence. Leukemia. 2017;31(4):896–902.
Thapaliya P, et al. International working group for myelofibrosis research and treatment response assessment and long-term follow-up of 50 myelofibrosis patients treated with thalidomide-prednisone based regimens. Am J Hematol. 2011;86(1):96–8.
Mesa RA, et al. Lenalidomide and prednisone for myelofibrosis: Eastern Cooperative Oncology Group (ECOG) phase 2 trial E4903. Blood. 2010;116(22):4436–8.
• Castillo-Tokumori F, et al. Retrospective analysis of the clinical use and benefit of lenalidomide and thalidomide in myelofibrosis. Clin Lymphoma Myeloma Leuk. 2020;20(12):e956–60. This is a more contemporary retrospective study demonstrating response rates for immunomodulatory drugs when used to treat MF anemia.
• Oh ST, et al. ACVR1/JAK1/JAK2 inhibitor momelotinib reverses transfusion dependency and suppresses hepcidin in myelofibrosis phase 2 trial. Blood Adv. 2020;4(18):4282–91. This study provides some proof of principle regarding the ability of momelotonib to suppress hepcidin and offer anemia responses in some patients with MF and transfusion-dependent anemia.
Asshoff M, et al. Momelotinib inhibits ACVR1/ALK2, decreases hepcidin production, and ameliorates anemia of chronic disease in rodents. Blood. 2017;129(13):1823–30.
Mesa RA, et al. SIMPLIFY-1: a phase III randomized trial of momelotinib versus ruxolitinib in Janus kinase inhibitor-naive patients with myelofibrosis. J Clin Oncol. 2017;35(34):3844–50.
Harrison CN, et al. Momelotinib versus best available therapy in patients with myelofibrosis previously treated with ruxolitinib (SIMPLIFY 2): a randomised, open-label, phase 3 trial. Lancet Haematol. 2018;5(2):e73–81.
Gerds AT, et al. A phase 2 study of luspatercept in patients with myelofibrosis-associated anemia. Blood. 2019;134(Supplement_1):557–557.
Gerds AT, et al. Duration of response to luspatercept in patients (Pts) requiring red blood cell (RBC) transfusions with myelofibrosis (MF) - updated data from the phase 2 ACE-536-MF-001 Study. Blood. 2020;134(Supplement_1):557 ((557)).
Kleppe M, et al. Dual targeting of oncogenic activation and inflammatory signaling increases therapeutic efficacy in myeloproliferative neoplasms. Cancer Cell. 2018;33(1):29-43 e7.
Talpaz Mea. CPI-0610, a bromodomain and extraterminal domain protein (BET) inhibitor, as monotherapy in advanced myelofibrosis patients refractory/intolerant to JAK inhibitor: update from phase 2 MANIFEST Study. Blood. 2020. 134(Paper 2163).
Verstovsek S et al. CPI-0610, bromodomain and extraterminal domain protein (BET) inhibitor, as “add-on” to ruxolitinib, in advanced myelofibrosis patients with suboptimal response: update of MANIFEST Phase 2 Study. Blood. 2020(Supplement_1): p. Abstract 56.
Mascarenhas J et al. CPI-0610, a bromodomain and extraterminal domain protein (BET) inhibitor, in combination with ruxolitinib, in JAK-inhibitor-naïve myelofibrosis patients: update of MANIFEST Phase 2 Study. Blood. 2020(Supplement_1): p. Abstract 55.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The author declares no competing interests.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by the author.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Myeloproliferative Neoplasms
Rights and permissions
About this article
Cite this article
Stein, B.L. Management of Myelofibrosis-Associated Anemia: Focus on Standard Agents and Novel Therapeutics in Phase 3 Clinical Trials. Curr Hematol Malig Rep 16, 483–489 (2021). https://doi.org/10.1007/s11899-021-00651-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11899-021-00651-3