Abstract
Purpose of Review
Myocardial metabolism is intricately linked to cardiac function. Perturbations of cardiac energy metabolism result in an energy-starved heart and the development of contractile dysfunction. In this review, we discuss alterations in myocardial energy supply, transcriptional changes in response to different energy demands, and mitochondrial function in the development of heart failure.
Recent Findings
Recent studies on substrate modulation through modifying energy substrate supply have shown cardioprotective properties. In addition, large cardiovascular outcome trials of anti-diabetic agents have demonstrated prognostic benefit, suggesting the importance of myocardial metabolism in cardiac function.
Summary
Understanding molecular and transcriptional controls of cardiac metabolism promises new research avenues for metabolic treatment targets. Future studies assessing the impact of substrate modulation on cardiac energetic status and function will better inform development of metabolic therapies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A salient feature of the heart is its metabolic flexibility [1], allowing it to efficiently adapt to varying ATP demands through utilisation of multiple energy substrates such as fatty acids, glucose, lactate, ketones, and amino acids. This is achieved through a complex network of interacting metabolic pathways involving each class of energy substrate, which in turn is capable of remodelling in chronic pathophysiological conditions [2]. As cardiac function is intricately linked to its metabolism, alterations in metabolic pathways can lead to morphological and functional changes. This is seen most clearly in heart failure, where metabolic inflexibility and energetic deficit are associated with maladaptive metabolic remodelling [3, 4].
This review aims to provide an overview of cardiac metabolism in heart failure at its three most important stages: [1] substrate utilisation, [2] ATP generation, and [3] ATP transport and stores. The role of mitochondrial function in the development of heart failure, the importance of transcriptional and post-translational modification in the regulation of cardiac metabolism, and an overview of how perturbations in this tightly regulated system can lead to the development of cardiac dysfunction are included.
Cardiac Metabolism in the Normal Heart
Step 1: Substrate Uptake and Metabolism
The first stage of cardiac metabolism is substrate delivery, uptake, and generation of acetyl-CoA to facilitate entry into the tricarboxylic acid (TCA) cycle [5, 6•]. Under basal conditions, the majority of acetyl-CoA formation is from long-chain fatty acyl-coenzyme A (60–90%) and pyruvate (10–40%, derived equally from glycolysis and lactate) [7, 8]. Alternative sources such as ketone bodies and amino acids form a minor source of ATP generation under normal physiological conditions.
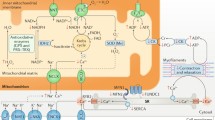
The selection of energy substrate for ATP production depends on substrate availability, energy demands, and the prevailing metabolic and hormonal conditions. Each energy substrate pathway is regulated by rate-limiting reactions, which in return respond to feedback or feedforward loops, and end-product inhibition as well as energy ‘sensors’ such as AMP-activated protein kinase (AMPK). One example of this feedback inhibition is demonstrated by the reciprocal relationship between myocardial fatty acid and glucose metabolism (Randle cycle), where accumulation of acetyl-CoA results in the activation of pyruvate dehydrogenase kinase (PDK4) and inhibition of pyruvate oxidation [9]. Figure 1 summarises the metabolic pathways corresponding to each energy substrate of the myocardium [7, 10].
Overview of energy metabolism pathways in a normal heart. Fatty acids are transported into the cardiomyocyte via CD36 and fatty acid transporter protein (FAT) and undergo esterification to form fatty acyl-CoA. The acyl group is transferred to carnitine by carnitine palmitoyltransferase 1 (CPT-1) to form long-chain acylcarnitine and is transported into the mitochondria where it is converted back to fatty acyl-CoA by CPT-2. The fatty acyl-CoA then undergoes beta-oxidation to form acetyl-CoA. The activity of CPT1 is regulated by malonyl-CoA which is formed through carboxylation of acetyl-CoA by acetyl-CoA carboxylase (ACC) and reverted to acetyl-CoA by malonyl-CoA decarboxylase (MCD). The activity of ACC is regulated by phosphorylation via energy sensors such as AMP-activated protein kinase (AMPK). Glucose enters the cardiomyocyte through glucose-transporters-1 or 4 (GLUT-1, GLUT-4). Free glucose is phosphorylated into glucose-6-phosphate (glucose-6P), which can enter different pathways such as glycolysis, pentose phosphate pathway, or the hexosamine biosynthesis pathway. Glycolysis remains the main pathway, producing pyruvate, reduced nicotinamide adenine dinucleotide (NADH), and a small amount of ATP [122]. Lactate is transported into the cardiomyocytes via the monocarboxylic acid transporter (MCT1) and converted into pyruvate by lactate dehydrogenase (LDH), a process consuming reduced nicotinamide adenine dinucleotide (NADH). Pyruvate enters the mitochondria via the mitochondrial pyruvate carrier (MPC) and is converted into acetyl-CoA by pyruvate dehydrogenase (PDH). PDH is inhibited by phosphorylation through pyruvate dehydrogenase kinase isoenzyme 4 (PDK4). Pyruvate can also undergo carboxylation to form malate or oxaloacetate to replenish the TCA cycle (anaplerosis) [123]. Ketones are taken up into the cardiomyocyte by monocarboxylate transporter 1 (encoded by SLC16A gene) and undergo a series of reactions mediated by β-hydroxybutyrate dehydrogenase 1 (BDH1), succinyl-CoA:3 oxoacid-CoA transferase (SCOT), and acetyl-CoA acyltransferase (ACAT1) to form acetyl-CoA. Branched-chain amino acids (BCAA) are transported into the cardiomyocyte via the branched-chain amino acid:cation symporter family (LIVCS). It is converted into branched-chain ketoacids (BCKA) by the mitochondrial branched-chain aminotransferase (BCATm) and forms acetyl-CoA and succinyl-CoA via oxidative decarboxylation by branched-chain alpha-ketoacid dehydrogenase (BCKDH). The activity of BCKDH is regulated via phosphorylation by BCKDH kinase and dephosphorylation through protein phosphatase C2m (PPC2m). CACT, carnitine-acylcarnitine translocase
Step 2: ATP Generation via Oxidative Phosphorylation
NADH and FADH2 produced from the TCA cycle and glycolysis donate electrons to the respiratory chain, releasing free energy which is used to create an electrochemical proton gradient across the mitochondrial inner membrane. The gradient drives ATP production by F1/F0 ATP synthase [11]. Under aerobic conditions, this contributes to >95% of ATP produced. The rate of oxidative phosphorylation is regulated by ADP and mitochondrial Ca2+ homeostasis. In a state of increased ATP demand, there is an increase in cytosolic Ca2+ flux, promoting mitochondrial Ca2+ uptake. This is crucial in regenerating reduced pyridine nucleotides through increased TCA cycle activity [12].
Step 3: ATP Shuttling and Buffering: the Creatine Kinase System
The final step in cardiac energy metabolism involves the shuttling of ATP to cellular regions of high ATP demand. This link between energy production and energy utilisation is provided by the creatine kinase (CK) system. The CK reaction consists of a phosphate donor (phosphocreatine, PCr), a phosphate acceptor (creatine, Cr), and the catalytic enzyme, CK, as follows:
This generates phosphocreatine (PCr) which is comparatively smaller and less polar than ATP, allowing rapid diffusion from the mitochondria into the cytosol. The reverse reaction is catalysed by the cytosolic CK, transferring the phosphoryl group back to ADP [13]. As such, the CK system buffers ATP levels in settings of fluctuating demand [14]. As the CK equilibrium constant favours ATP production by nearly 100-fold, this maintains ATP levels in most situations except in advanced heart failure [15, 16].
Energy ‘Sensors’
AMP-activated protein kinase (AMPK) and mechanistic target of rapamycin (mTOR) are evolutionarily conserved kinases that regulate cellular metabolism through sensing energy and nutrient levels. The activation of AMPK is dependent on intracellular energy levels, specifically an increased AMP/ATP ratio, as well as upstream kinases such as liver kinase B1 (LKB1) and calcium/calmodulin-dependent protein kinase (CAMKK) [17, 18]. AMPK activation promotes catabolic pathways such as glucose and fatty acid (FA) oxidation and inhibits anabolic processes (e.g. protein synthesis) mediated by mTOR signalling. AMPK also regulates signalling pathways controlling mitochondrial biogenesis and autophagy [19].
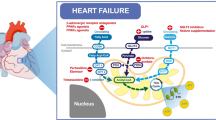
Energy ‘sensing’ and control of mitochondrial metabolism are also achieved through post-translational modification. The sirtuin family (SIRT), a group of NAD+-dependent histone deacetylase (HDAC), in particular the nuclear SIRT1 and mitochondrial SIRT3 regulate multiple cellular pathways (Fig. 2) through interactions with protein kinases such as LKB1, AMPK, and PGC1α [20]. More recently, SIRT1 activation with resveratrol reversed cardiac remodelling and improved function in an animal model of diabetic cardiomyopathy [21] and dilated cardiomyopathy [22].
Overview of AMP-kinase, sirtuin-1 (SIRT1), peroxisome proliferator-activated receptors (PPARs), and PPAR-gamma coactivator (PGC-1α) pathways. The mTOR signalling pathway regulates multiple anabolic cellular processes, including protein synthesis, cellular proliferation, cell metabolism, and autophagy. CAMKK, calcium/calmodulin-dependent protein kinase 2; LKB1, liver kinase B1; NF-kB, nuclear factor kappa B; MnSOD, manganese superoxide dismutase; eNOS, endothelial nitric oxide synthase; ERR, oestrogen-related receptors; NRF, nuclear-related factor; ULK1, Unc-51-like autophagy activating kinase; mTOR, mechanistic target of rapamycin, PFK, phosphofructokinase
Transcriptional Regulation of Metabolic Pathways
Central to metabolic transcriptional remodelling in heart failure are the peroxisome proliferator-activated receptors (PPARs), a network of nuclear transcription factors crucial for fatty acid metabolism and mitochondrial biogenesis. Of the three isoforms (α, δ, and γ), PPARα and PPARδ are highly expressed within cardiac myocytes. PPARα increases fatty acid uptake, oxidation, and ketogenesis [23], and its overexpression produces metabolic phenotypes mimicking insulin resistance [24, 25]. Conversely, PPARδ plays a balanced role in increasing both FA and glucose utilisation, as well as regulating the reactive oxygen species (ROS) scavenging system [26, 27]. PPARγ is expressed at very low levels in cardiomyocytes and regulates intracellular lipid trafficking [28].
A pivotal transcriptional coregulator within cardiomyocytes is the PPAR-gamma coactivator (PGC-1α) which responds to a wide range of stimuli including starvation, exercise, and change in substrate availability. The downstream effector pathways of PGC1α are summarised in Fig. 2 and include PPARα, ERRα, and nuclear respiratory factor 1 (NRF1) [29].
Cardiac Metabolism in Heart Failure
Heart failure is associated with derangement in all three fundamental steps of energy generation. Many single-gene transgenic, gain- and loss-of-function animal models have demonstrated a wide array of altered substrate utilisation with conflicting outcomes on contractile function and remodelling [1, 5, 6•]. This could reflect the differing pathophysiology of cardiac diseases, duration, and severity of disease. The main questions remain - is the metabolic remodelling an adaptive or maladaptive response to external stimuli? Is an altered energetic state the cause or consequence of heart disease?
Substrate Utilisation in Heart Failure
Metabolic remodelling and the ‘loss of metabolic flexibility’ are believed to antedate, initiate, and sustain structural and functional remodelling in the development of heart failure. Specifically, reversion to foetal gene expression, impairment of FA oxidation (FAO) capacity, and insufficient compensatory increases in glucose metabolism are thought to underlie the transition to heart failure.
Fatty Acid Metabolism
Animal models of altered fatty acid metabolism in the pathophysiology of heart failure have been inconsistent, showing fatty acid oxidation is either decreased [30, 31], unchanged [32, 33], or increased [34]. This may be due to differences between animal models, disease aetiology, and stage of disease. Whether these changes represent an adaptive or maladaptive response remains unresolved.
A key observation in pathological hypertrophy and heart failure is the downregulation of PPAR α, an important transcriptional regulator of lipid metabolism [35]. PPARα knockout (KO) mice demonstrate severely impaired FAO and increased cardiac glucose oxidation but fail to maintain contractile performance at high workload with increased myocardial fibrosis [36, 37]. Conversely, increased PPARα expression in transgenic mice increases fatty acid uptake, assimilating a phenotype similar to that of diabetic or obese cardiomyopathy [38]. However, increasing both glucose and fatty acid metabolism, thus overall myocardial oxidative metabolism, through PPARβ/δ overexpression appears cardioprotective against mechanical stress [39].
Dysregulation of specific pathways of fatty acid metabolism also appears to have deleterious effects. Interruption in fatty acid uptake alone through deletion of CD36 or carnitine palmitoyltransferase-1 (CPT-1) exacerbated cardiac hypertrophy and cardiac dysfunction in animal models [40, 41]. Aberration in fatty acid uptake and its association with pathological hypertrophy and heart failure is further supported by the observation of a high prevalence of CD36 deficiency in patients with dilated cardiomyopathy [42].
Despite this, clinical trials suggest further inhibition of fatty acid oxidation in order to recouple glycolysis to glucose oxidation may be beneficial. These include partial fatty acid oxidation inhibitors such as trimetazidine [43] and ranolazine [44] as well as CPT-1 inhibitors, e.g. perhexiline [45]. Of note, these agents may exert its beneficial effects through pathways beyond that of metabolic alteration (e.g. inhibition of late inward sodium channels by ranolazine) and these studies remain limited by their small sample size. Some animal models of malonyl-CoA inhibition have also shown promise but this has not been studied in humans [46].
The effects of increasing FAO on cardiac remodelling have also been investigated. The specific upregulation of mitochondrial FAO through deletion of ACC2 appears protective against cardiac hypertrophy in ACC2-KO mice [47]. While animal models of PPARα agonism have shown variable results [48], there have been no clinical trials specifically studying the effect of PPARα modulation on heart failure outcomes. However, post hoc analyses of a large dyslipidaemia and diabetes trial have shown reduction in heart failure hospitalisation in patients treated with PPARα agonists, e.g. fenofibrate [49, 50•].
Overall, these studies suggest the importance of balancing fatty acid availability, uptake, and oxidation in maintaining normal cardiac function. Certainly, preservation of myocardial capacity for FAO appears cardioprotective to a degree, especially in context of haemodynamic stress. Thus, over-reliance on one energy substrate regardless of which substrate this may be and the inability to utilise another would seem a maladaptive response in heart failure.
Glucose Metabolism
The majority of animal models of a failing heart, whether subjected to pressure-overload or pacing-induced cardiomyopathy, have demonstrated the uncoupling between glycolysis and glucose oxidation. The shift towards glucose utilisation in context of the failing heart is thought to improve myocardial ‘efficiency’ by producing more ATP per molecule of oxygen consumed [51].
However, considering that fatty acids remain a very effective carbon source for high volume of ATP production and are abundantly available, does increased glucose reliance (and subsequently impaired FAO) contribute to the development of cardiac dysfunction? PPARα nulled hearts fail to maintain myocardial energetic demands in the presence of high cardiac workload, developing cardiomyopathy at old age. This is interestingly reversed through the overexpression of GLUT1[37], proposing a theory that the inherent capacity of the adult myocardium to increase glucose oxidation in context of decreased FAO capacity may not provide sufficient ATP under stress. In addition, the unmatched rise in glycolytic rates cause build-up of glycolysis-derived protons, intracellular acidosis, and decreased cardiac contractility due to inhibition of slow Ca2+ current [52].
Therefore, it is reasonable to propose that increasing both glucose uptake and oxidation capacity may improve myocardial energetics. Studies of glucose-insulin-potassium (GIK) infusions and glucagon-like peptide (GLP-1) agonists which aim to increase insulin secretion, sensitivity, and glucose uptake have suggested an improvement in cardiac function in patients with ischaemic cardiomyopathy in settings of an acute myocardial infarction [53, 54]. Similarly, inhibition of PDH kinase (PDHK) with dichloroacetate (DCA) appears to improve haemodynamic status (e.g. stroke volume and myocardial work) in patients with coronary artery disease and congestive heart failure [55, 56].
Carbohydrate vs. Fatty Acid
Animal models mimicking metabolic shifts towards a specific substrate, e.g. glucose only or fatty acid only have provided further insight. Cardiac-specific overexpression of PDK4 leading to increased FAO and decreased glucose metabolism was not detrimental to cardiac function following an ischaemic insult [57], suggesting increased FAO alone may not be key to abnormal cardiac remodelling.
Substrate modification on GLUT-1 transgenic mice shed further light on the importance of preserving a balanced metabolic profile. When exposed to a high-fat diet, GLUT-1 transgenic mice were observed to maintain a high glucose oxidation rate (unlike control animals) suggesting longer-term transcriptional downregulation of fatty acid metabolism in these transgenic mice. Importantly, this was accompanied by increased oxidative stress and development of contractile dysfunction [58].
This proposes that the focus of therapeutic targets of metabolism should be that of sustaining flexibility of substrate utilisation, rather than ‘preferencing’ one over another. More recently, the cardioprotective role of substrate manipulation has been explored in surgical post-coronary revascularisation. Intralipid infusion post-sternotomy as a pre-conditioning agent was associated with reduced need for high-dose inotropic support, earlier normalisation of serum troponin levels, and higher values of cardiac index measured by invasive cardiac monitoring [59]. These effects are believed to be mediated by phosphorylation of glycogen synthase kinase-3β (GSK-3β) and delaying of the opening of the mitochondrial permeability transition pore (mPTP) associated with cellular apoptosis [60]. The effect of substrate modulation and metabolic flexibility has been further studied with magnetic resonance spectroscopy, where intralipid infusion was seen to improve myocardial energetics (PCr/ATP ratio) and cardiac contractility in patients with established non-ischaemic dilated cardiomyopathy [61]. This represents an exciting new approach to manipulating metabolism for improved cardiac function.
Ketone Body Metabolism
Ketone bodies (β-hydroxybutyrate (BOHB) and acetoacetate) are produced by the liver during periods of fasting. Upon entering cardiomyocytes and the mitochondrial matrix, ketone bodies form acetyl-CoA through a series of reactions catalysed by BOHB dehydrogenase (BDH1), succinyl-CoA:3-oxoacid-CoA-transferase (SCOT), and acetyl-CoA acetyltransferase (ACAT1) within the mitochondria. Under normal circumstances, ketone bodies account for 5–10% of the myocardial energy substrate metabolism [62]. Serum ketone levels are the major determinant of myocardial ketone body oxidation rate.
Circulating ketone levels are reportedly increased in patients with heart failure [63]. In parallel, BDH1 and SCOT expressions are upregulated in animal and human models of heart failure, supporting the notion of increased reliance on ketone body utilisation in a diseased state [64]. However, the same question applies—is this increased reliance an adaptive or maladaptive response? Gene-targeted mouse models suggest that increased myocardial ketone body oxidation is adaptive; SCOT-KO mice exhibit accelerated pathological remodelling, mitochondrial dysfunction, and exacerbated cardiac dysfunction in context of pressure-overload [65]. Conversely, cardiac-specific BDH1 overexpression had enhanced antioxidant enzyme expression and was resistant to contractile dysfunction when subjected to pressure-overload stimuli [66].
Nevertheless, ketone body utilisation has a reciprocal relationship with glucose and fatty acid metabolism. Ketone body infusion in healthy human volunteers was associated with increased myocardial blood flow but decreased myocardial glucose uptake on positron emission tomography, an effect due to interrupted GLUT4 translocation [67, 68]. BOHB also appears to inhibit myocardial fatty acid uptake, through a mechanism independent of malonyl-CoA levels [69].
The consequences of this inhibitory effect of ketone bodies on myocardial glucose and fatty acid uptake require further characterisation. However, stimulating ketone oxidation appears to improve cardiac function. Increasing ketone body availability through either intravenous ketone infusions or oral ketone esters has beneficial impact on cardiac haemodynamics and function measured by LVEF [70•, 71]. Sodium-glucose-linked transporter 2 (SGLT2i) inhibitors may exert cardioprotective effects through increasing circulating ketone bodies to an ‘energy-starved’ failing heart [72]. This hypothesis is supported by a non-diabetic porcine heart failure model where empaglifozin led to a switch towards ketone body and fatty acid utilisation and amelioration of adverse cardiac remodelling [73•].
Branched-Chain Amino Acids (BCAA) Metabolism
Branched-chain amino acids (BCAA) such as leucine, isoleucine, and valine account for <2% of ATP production in a normal heart under physiological conditions [74]. These enter the cell through specialised amino acid transporters regulated by substrate availability [75]. BCAAs undergo transamination (by the mitochondrial branched-chain amino-transaminase, BCATm) to form corresponding branched-chain alpha-ketoacids (BCKA). The rate-limiting step of BCAA metabolism is the subsequent oxidative decarboxylation of BCKAs by the branched-chain alpha-keto acids dehydrogenase complex (BCKDH) to form either acetyl-CoA for the TCA cycle or succinyl-CoA for anaplerosis. This step is regulated by phosphorylation (inhibition) and dephosphorylation (activation) of BCKDH by either BCKDH kinase or protein phosphatase C2m (PPC2m), respectively [76].
Animal and human models of the failing heart demonstrate impaired BCAA oxidation, increased BCAA and BCKA levels, and decreased expression of enzymes involved in BCAA metabolism [77, 78]. Although less significant in energy generation, accumulation of BCAA and BCKA promotes cardiac hypertrophy through persistent mTOR signalling and cardiac insulin resistance [79, 80, 81]. In addition, accumulation of BCKA is believed to disrupt mitochondrial redox homeostasis through significantly increased superoxide production. Importantly, these effects are reversed with enhanced BCAA oxidation and inhibition of mTOR signalling with rapamycin in mouse models [77].
These findings have stimulated research interests in developing therapeutic strategies targeting BCAA metabolism in heart failure. Inhibition or stimulation of BCATm is limited by the unintended consequence of preferentially increasing BCAA or BCKA respectively. Inhibition of BCKDH kinase with BT2 (3,6-dichlorobenzo[b]thiophene-2-carboxylic acid), thus decreased BCAA and BCKA levels, in a pressure-overload mouse model ameliorated negative cardiac remodelling and dysfunction; however, there are no human trials to date [82]. More recently, dietary interventions in animal and human models suggest the potential of improving BCAA metabolism and insulin sensitivity, providing yet another metabolic target for heart failure treatment [83, 84].
Mitochondrial Dysfunction in Heart Failure
In addition to a loss of metabolic flexibility, heart failure is associated with derangement in mitochondrial function resulting in mechano-energetic uncoupling; these include abnormal mitochondrial structure and function, increased production of reactive oxygen species (ROS), altered mitochondrial ion homeostasis, and impairment in ATP generation. Figure 3 provides an overview of mitochondrial function.
Overview of mitochondrial function including ATP generation, redox signalling, and calcium handling. In a healthy cardiomyocyte, the ryanodine receptor (RyR2) of the sarcoplasmic reticulum (SR) is in close proximity with the mitochondria, where the mitochondrial calcium uniporter (MCU) is located. This facilitates a specific concentration of free Ca2+ in the SR-mitochondria interface to enable mitochondrial uptake of cytosolic Ca2+, a process that sustains mitochondrial metabolism for cardiomyocyte contraction. Mitochondrial Ca2+ efflux is mediated by the mitochondrial Na+/Ca2+ exchanger (NCLX) located near the sarcoplasmic reticulum Ca2+ ATPases (SERCA). Cytosolic Ca2+ levels are also regulated by other transporters in the plasma membrane such as the sodium-calcium exchanger (NCX), Ca2+ ATPases, and sodium-hydrogen exchanger (NHE). The mitochondrial permeability transition pore (mPTP) located in the inner mitochondrial membrane is normally closed and opens when stimulated by mitochondrial Ca2+ overload—this results in mitochondrial osmotic swelling and dissipation of the mitochondrial inner membrane potential, ultimately resulting in cell death. Reactive oxygen species such as superoxide (O2−) are produced at complex I and III of the electron transport chain and are converted to H2O2 by manganese superoxide dismutase (MnSOD). Mitochondrial H2O2 is converted to H2O by catalase and mitochondrial-localised glutathione peroxidases (GPx1), consuming reducing equivalents such as NADPH. ANT, adenine nucleotide translocase; NNT, nicotinamide nucleotide transhydrogenase. *Figures 1, 2, and 3 were created with BioRender.com
Abnormal Mitochondrial Structure and Function
Mitochondria in heart failure undergo several structural changes [85, 86] that reduce mitochondrial respiratory capacity in animal and human models of heart failure. In particular, the expression of a key phospholipid within the inner mitochondrial membrane, cardiolipin, appears to be reduced in heart failure [87, 88].
Cardiolipin functions as a key co-factor for mitochondrial transport proteins, including the respiratory supercomplexes (complex I, III, IV and V). and plays a role in the retention of cytochrome c, a key regulator of cellular apoptosis within the inner mitochondrial membrane. ROS-mediated oxidation of cardiolipin results in release of cytochrome c into the cytoplasm and activation of caspase-3. The importance of cardiolipin is exemplified in genetic disorders that affect cardiolipin synthesis such as Barth syndrome (BTHS) and dilated cardiomyopathy with ataxia (DCMA) [89]. Cardiolipin levels in heart failure are also reduced, leading to increased production of ROS, peroxidation of cardiolipin, mitochondrial dysfunction, and cellular death [90].
Increased ROS Production
ROS production is low under physiological conditions and kept in check by endogenous scavenging systems. Mitochondria in the failing heart are associated with an increased production of ROS, beyond the capacity of the endogenous ROS scavenging system [91, 92]. Elevated mitochondrial ROS production results in mitochondrial DNA damage, defect in the electron transport chain ultimately triggering cell death cascades. As such, targeting ROS with antioxidant therapies would seem a promising approach in treatment of cardiovascular disease. Nevertheless, trials with non-specific antioxidants such as vitamin E had no effect on major adverse cardiovascular events, or indeed hospitalisation for heart failure [93]. Possible barriers to this include the untargeted treatment as well as lack of cell permeability. Although targeted antioxidants such as mitoquinone (MitoQ) have demonstrated cardioprotective effects in animal models of pressure-overload and ischaemia-reperfusion injury, this has not been trialled in patients with heart failure to date [94, 95].
Altered Calcium Handling
Mitochondrial calcium handling is closely linked to its role in excitation-contraction coupling, ATP synthesis and cellular death. The increased demand for ATP in conditions of high workload is facilitated by a matched rise in the rate of ADP phosphorylation through acceleration in the TCA cycle. This is achieved by accumulation of calcium (Ca2+) within the mitochondrial matrix. Mitochondria also function as a reservoir and are able to accumulate a large amount of Ca2+ [96]. This is regulated by the close proximity between the mitochondrial Ca2+ uniporter protein (MCU) in the inner mitochondrial membrane (IMM) and the sarcoplasmic reticulum (SR), as well as the mitochondrial Na+/Ca2+ exchanger (NCLX), which facilitates Ca2+ efflux [97].
In a failing heart, the function of these membrane-bound pumps for calcium handling is affected due to its energy and ROS dependence. Cellular calcium handling is deranged due to increased ROS production and intracellular sodium levels in heart failure [98, 99]. Reduced expression of cardiac SR calcium ATPase (SERCA2a) also contributes to increased end-diastolic cytosolic calcium level and prolongation of calcium transient during diastole, causing impaired diastolic relaxation in heart failure with preserved ejection fraction (HFpEF) [100].
Targeting therapies that augment mitochondrial Ca2+ uptake or prevent Ca2+ extrusion may prove beneficial considering the role of Ca2+ in replenishing the endogenous ROS scavenging system. Selective NCLX inhibition with CGP-37157 in a guinea pig model of heart failure and sudden cardiac death preserved cardiac contractility and prevented fatal arrhythmias [101]. To date, this has not been trialled in humans. A selective inhibitor of NHE type I, cariporide in ischaemia/reperfusion injury, was unfortunately associated with increased mortality in patients undergoing high-risk coronary artery bypass surgery, despite reducing the rate of myocardial injury [102, 103].
Conversely, mitochondrial Ca2+ overload precipitates the opening of a high-conductance channel within the IMM—the mitochondrial permeability transition pore (MPTP). Opening of MPTP is associated with loss of IMM potential (and thus uncoupling of oxidative phosphorylation), mitochondrial dysfunction, and subsequent cellular death. Cardiomyocytes from animal models of heart failure demonstrate an increased tendency for mPTP opening and decreased mitochondrial membrane potential, with inhibition of MPTP using cyclosporin ameliorating mitochondrial dysfunction [104].
Abnormalities in Electron Transport Chain (ETC)
Impaired oxidative phosphorylation secondary to ETC abnormalities has also been reported, including reduced number and function of the respiratory complexes [105, 106] and reduced coenzyme Q (CoQ) levels. CoQ functions as a redox-cycling coenzyme within the ETC, with the ability to accept or donate an electron depending on its redox potential. In line with this, the Q-SYMBIO trial showed CoQ supplementation led to symptomatic improvement and reduced major adverse cardiovascular events in heart failure with reduced ejection fraction [107].
Energy Buffering and Transfer in Heart Failure
The creatine kinase (CK) system is a crucial component of the metabolic machinery, especially in organ tissues with high and intermittent energy fluctuations such as the heart. It serves as a ‘temporal’ energy buffer, maintaining adequate ATP concentration according to energy demand, and a ‘spatial’ buffer tightly coupling ATP-producing and consuming processes. Creatine (Cr) is absorbed from dietary sources and synthesised endogenously in a two-stage process. Transport of Cr into myocytes is mediated by a Na+/Cl- dependent transporter (CrT; SLC6A8) and is largely regulated by substrate availability and cellular metabolite state [108].
A reduction in PCr/ATP ratio is well-documented across a range of cardiac pathology [109]. Additionally, a lower PCr/ATP ratio is correlated with a higher NYHA class, lower left ventricular ejection fraction, and a worse prognosis [109]. The initial decrease in PCr/ATP reflects a decrease in PCr levels as ATP concentrations are seen to decrease only at advanced stages of HF [110]. Of note, a pseudonormalised PCr/ATP ratio may be achieved when ATP is depleted at later stages of disease, thus underestimating changes in myocardial energetic state [111].
The rightward shift in CK equilibrium (towards ATP generation) in circumstances of chronically increased workload and a reduction in total myocardial creatine content in a failing heart likely contribute to the decreased PCr/ATP ratio [112]. Creatine pool size is positively correlated with left ventricular ejection fraction in dilated cardiomyopathy and inversely related to plasma NTproBNP levels [113]. Whether creatine deficiency (and thus decreased PCr) contributes to the development of heart failure, however remains unclear.
Animal models on the causal role of a dysregulated CK system in chronic heart failure remain conflicting. Reduced creatine levels (either pharmacologically through beta-guanidinopropionate or genetic knockout of guanidinoacetate methyltransferase, GAMT) and loss of CK activity in models of chronic heart failure have little consequence on LV function or survival [114]. Nevertheless, a functioning CK system remains important for preserved contractile reserve in context of acute insults or increased workload such as myocardial infarction, although not at rest [115–117]. Indeed, overexpression of plasma membrane CrT in a mouse model of ischaemia/reperfusion injury suggests increased myocardial creatinine levels protect against myocardial necrosis and adverse LV remodelling, supporting the role of improved energy reserve particularly in acute stress [118].
Importantly, some evidence has suggested augmentation of the CK system is cardioprotective in ischaemia/reperfusion injury [119] and heart failure [120]. Moreover, myocardial CK flux remained a significant predictor of heart failure outcomes, independent of NYHA class and LVEF suggesting the role of altered ATP kinetics in risk stratification of HF [121]. Further research to better understand the importance of CK system enhancement will help identify novel heart failure therapies.
Conclusion
Metabolic changes that occur in heart failure are complex. The numerous animal models attempting to explore these are challenged by the ability to accurately recapitulate disease aetiology, duration, and severity, and as such have provided conflicting information. Nevertheless, there is consistency in the description of transcriptional changes leading to altered cardiac metabolism in the failing heart. This ultimately manifests in the energy-starvation hypothesis described in heart failure. Future studies identifying optimal strategies to restore metabolic flexibility will advance the development of targeted metabolic therapy in heart failure.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Neubauer S. The failing heart — an engine out of fuel. N Engl J Med. 2007;356(11):1140–51.
Saddik M, Lopaschuk GD. Myocardial triglyceride turnover and contribution to energy substrate utilization in isolated working rat hearts. J Biol Chem. 1991;266(13):8162–70.
Karwi QG, Uddin GM, Ho KL, Lopaschuk GD. Loss of metabolic flexibility in the failing heart. Front Cardiovasc Med. 2018;6(5):68.
Razeghi P, Young ME, Alcorn JL, Moravec CS, Frazier OH, Taegtmeyer H. Metabolic gene expression in fetal and failing human heart. Circulation. 2001;104(24):2923–31.
Doenst T, Nguyen TD, Abel ED. Cardiac metabolism in heart failure. Circulation Research. 2013;113(6):709–24.
Lopaschuk GD, Karwi QG, Tian R, Wende AR, Abel ED. Cardiac energy metabolism in heart failure. Circulation Research. 2021;128(10):1487–513. This recent review article provides a comprehensive overview of alterations in cardiac metabolism in context of heart failure, including changes
Lopaschuk GD, Ussher JR, Folmes CDL, Jaswal JS, Stanley WC. Myocardial fatty acid metabolism in health and disease. Physiol Rev. 2010;90(1):207–58.
Stanley WC, Recchia FA, Lopaschuk GD. Myocardial substrate metabolism in the normal and failing heart. Physiological Reviews. 2005;85(3):1093–129.
Randle PJ, Garland PB, Hales CN, Newsholme EA. The glucose fatty-acid cycle its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. The Lancet. 1963;281(7285):785–9.
van der Vusse GJ, van Bilsen M, Glatz JFC. Cardiac fatty acid uptake and transport in health and disease. Cardiovascular Research. 2000;45(2):279–93.
Murphy MP. How mitochondria produce reactive oxygen species. Biochem J. 2009;417(Pt 1):1–13.
Ferrari R, Pedersini P, Bongrazio M, Gaia G, Bernocchi P, Di Lisa F, et al. Mitochondrial energy production and cation control in myocardial ischaemia and reperfusion. Basic Res Cardiol. 1993;88(5):495–512.
Schlattner U, Tokarska-Schlattner M, Wallimann T. Mitochondrial creatine kinase in human health and disease. Biochim Biophys Acta. 2006;1762(2):164–80.
Wallimann T, Wyss M, Brdiczka D, Nicolay K, Eppenberger HM. Intracellular compartmentation, structure and function of creatine kinase isoenzymes in tissues with high and fluctuating energy demands: the ‘phosphocreatine circuit’ for cellular energy homeostasis. Biochem J. 1992;281(Pt 1):21–40.
Ingwall JS, Weiss RG. Is the failing heart energy starved? Circulation Research. 2004;95(2):135–45.
Bittl JA, Ingwall JS. Reaction rates of creatine kinase and ATP synthesis in the isolated rat heart A 31P NMR magnetization transfer study. J Biol Chem. 1985;260(6):3512–7.
Arad M, Seidman CE, Seidman JG. AMP-activated protein kinase in the heart. Circulation Research. 2007;100(4):474–88.
Li X, Liu J, Lu Q, Ren D, Sun X, Rousselle T, et al. AMPK: a therapeutic target of heart failure—not only metabolism regulation. Biosci Rep. 2019;39(1):20181767.
Dyck JRB, Lopaschuk GD. AMPK alterations in cardiac physiology and pathology: enemy or ally? J Physiol. 2006;574(Pt 1):95–112.
Sundaresan NR, Samant SA, Pillai VB, Rajamohan SB, Gupta MP. SIRT3 is a stress-responsive deacetylase in cardiomyocytes that protects cells from stress-mediated cell death by deacetylation of Ku70. Mol Cell Biol. 2008;28(20):6384–401.
Ma S, Feng J, Zhang R, Chen J, Han D, Li X, et al. SIRT1 activation by resveratrol alleviates cardiac dysfunction via mitochondrial regulation in diabetic cardiomyopathy mice. Oxid Med Cell Longev. 2017;2017:4602715.
Chen Q, Zeng Y, Yang X, Wu Y, Zhang S, Huang S, et al. Resveratrol ameliorates myocardial fibrosis by regulating Sirt1/Smad3 deacetylation pathway in rat model with dilated cardiomyopathy. BMC Cardiovascular Disorders. 2022;22(1):17.
Dubois V, Eeckhoute J, Lefebvre P, Staels B. Distinct but complementary contributions of PPAR isotypes to energy homeostasis. J Clinical Investigation. 2017;127(4):1202.
Hamblin M, Friedman DB, Hill S, Caprioli RM, Smith HM, Hill MF. Alterations in the diabetic myocardial proteome coupled with increased myocardial oxidative stress underlies diabetic cardiomyopathy. J Mol Cell Cardiol. 2007;42(4):884–95.
Jüllig M, Hickey AJ, Middleditch MJ, Crossman DJ, Lee SC, Cooper GJS. Characterization of proteomic changes in cardiac mitochondria in streptozotocin-diabetic rats using iTRAQTM isobaric tags PROTEOMICS. Clinical Applications. 2007;1(6):565–76.
Wang P, Liu J, Li Y, Wu S, Luo J, Yang H, et al. Peroxisome proliferator-activated receptor delta is an essential transcriptional regulator for mitochondrial protection and biogenesis in adult heart. Circ Res. 2010;106(5):911–9.
Burkart EM, Sambandam N, Han X, Gross RW, Courtois M, Gierasch CM, et al. Nuclear receptors PPARbeta/delta and PPARalpha direct distinct metabolic regulatory programs in the mouse heart. J Clin Invest. 2007;117(12):3930–9.
Son NH, Park TS, Yamashita H, Yokoyama M, Huggins LA, Okajima K, et al. Cardiomyocyte expression of PPARγ leads to cardiac dysfunction in mice. J Clin Invest. 2007;117(10):2791–801.
Fernandez-Marcos PJ, Auwerx J. Regulation of PGC-1α, a nodal regulator of mitochondrial biogenesis. Am J Clin Nutr. 2011;93(4):884S – 90.
Rosenblatt-Velin N, Montessuit C, Papageorgiou I, Terrand J, Lerch R. Postinfarction heart failure in rats is associated with upregulation of GLUT-1 and downregulation of genes of fatty acid metabolism. Cardiovasc Res. 2001;52(3):407–16.
Kato T, Niizuma S, Inuzuka Y, Kawashima T, Okuda J, Tamaki Y, et al. Analysis of metabolic remodeling in compensated left ventricular hypertrophy and heart failure. Circulation Heart Failure. 2010;3(3):420–30.
Taylor M, Wallhaus TR, Degrado TR, Russell DC, Stanko P, Nickles RJ, et al. An evaluation of myocardial fatty acid and glucose uptake using PET with [18F]fluoro-6-thia-heptadecanoic acid and [18F]FDG in Patients with Congestive Heart Failure. J Nucl Med. 2001;42(1):55–62.
Funada J, Betts TR, Hodson L, Humphreys SM, Timperley J, Frayn KN, et al. Substrate utilization by the failing human heart by direct quantification using arterio-venous blood sampling. PLOS ONE. 2009;4(10):e7533.
Voros G, Ector J, Garweg C, Droogne W, Van Cleemput J, Peersman N, et al. Increased cardiac uptake of ketone bodies and free fatty acids in human heart failure and hypertrophic left ventricular remodeling. Circulation Heart Failure. 2018;11(12):004953.
Sack MN, Rader TA, Park S, Bastin J, McCune SA, Kelly DP. Fatty acid oxidation enzyme gene expression is downregulated in the failing heart. Circulation. 1996;94(11):2837–42.
Loichot C, Jesel L, Tesse A, Tabernero A, Schoonjans K, Roul G, et al. Deletion of peroxisome proliferator-activated receptor-alpha induces an alteration of cardiac functions. Am J Physiol Heart Circ Physiol. 2006;291(1):H161-166.
Luptak I, Balschi JA, Xing Y, Leone TC, Kelly DP, Tian R. Decreased contractile and metabolic reserve in peroxisome proliferator–activated receptor-α–null hearts can be rescued by increasing glucose transport and utilization. Circulation. 2005;112(15):2339–46.
Finck BN, Lehman JJ, Leone TC, Welch MJ, Bennett MJ, Kovacs A, et al. The cardiac phenotype induced by PPARalpha overexpression mimics that caused by diabetes mellitus. J Clin Invest. 2002;109(1):121–30.
Liu J, Wang P, Luo J, Huang Y, He L, Yang H, et al. PPARβ/δ activation in adult hearts facilitates mitochondrial function and cardiac performance under pressure-overload condition. Hypertension. 2011;57(2):223–30.
Nakatani K, Masuda D, Kobayashi T, Sairyo M, Zhu Y, Okada T, et al. Pressure overload impairs cardiac function in long-chain fatty acid transporter CD36-knockout mice. Int Heart J. 2019;60(1):159–67.
He L, Kim T, Long Q, Liu J, Wang P, Zhou Y, et al. Carnitine palmitoyltransferase-1b deficiency aggravates pressure overload-induced cardiac hypertrophy caused by lipotoxicity. Circulation. 2012;126(14):1705–16.
Tanaka T, Sohmiya K, Kawamura K. Is CD36 Deficiency an etiology of hereditary hypertrophic cardiomyopathy? Journal of Molecular and Cellular Cardiology. 1997;29(1):121–7.
Gao D, Ning N, Niu X, Hao G, Meng Z. Trimetazidine: a meta-analysis of randomised controlled trials in heart failure. Heart. 2011;97(4):278–86.
Maier LS, Layug B, Karwatowska-Prokopczuk E, Belardinelli L, Lee S, Sander J, et al. JACC Heart Failure. 2013;1(2):115–22.
Lee L, Campbell R, Scheuermann-Freestone M, Taylor R, Gunaruwan P, Williams L, et al. Metabolic modulation with perhexiline in chronic heart failure: a randomized, controlled trial of short-term use of a novel treatment. Circulation. 2005;112(21):3280–8.
Dyck JRB, Hopkins TA, Bonnet S, Michelakis ED, Young ME, Watanabe M, et al. Absence of malonyl coenzyme A decarboxylase in mice increases cardiac glucose oxidation and protects the heart from ischemic injury. Circulation. 2006;114(16):1721–8.
Kolwicz SC, Olson DP, Marney LC, Garcia-Menendez L, Synovec RE, Tian R. Cardiac-specific deletion of acetyl CoA carboxylase 2 prevents metabolic remodeling during pressure-overload hypertrophy. Circulation Research. 2012;111(6):728–38.
Sarma S, Ardehali H, Gheorghiade M. Enhancing the metabolic substrate: PPAR-alpha agonists in heart failure. Heart Fail Rev. 2012;17(1):35–43.
Frick MH, Elo O, Haapa K, Heinonen OP, Heinsalmi P, Helo P, et al. Helsinki Heart Study primary-prevention trial with gemfibrozil in middle-aged men with dyslipidemia Safety of treatment, changes in risk factors, and incidence of coronary heart disease. N Engl J Med. 1987;317(20):1237–45.
Ferreira JP, Vasques-Nóvoa F, Ferrão D, Saraiva F, Falcão-Pires I, Neves JS, et al. Fenofibrate and heart failure outcomes in patients with type 2 diabetes: Analysis From ACCORD. Diabetes Care. 2022;45:1584. This post hoc analysis of a randomised controlled trial exploring the effect of the PPARα agonist, fenofibrate on heart failure hospitalisations, and cardiovascular death raises the need for prospective studies on the effect of PPARα agonists on cardiac remodelling and function.•
Eberli FR, Weinberg EO, Grice WN, Horowitz GL, Apstein CS. Protective effect of increased glycolytic substrate against systolic and diastolic dysfunction and increased coronary resistance from prolonged global underperfusion and reperfusion in isolated rabbit hearts perfused with erythrocyte suspensions. Circ Res. 1991;68(2):466–81.
Fiolet JWT, Baartscheer A. Cellular calcium homeostasis during ischemia; a thermodynamic approach. Cardiovascular Res. 2000;45(1):100–6.
Nikolaidis LA, Mankad S, Sokos GG, Miske G, Shah A, Elahi D, et al. Effects of glucagon-like peptide-1 in patients with acute myocardial infarction and left ventricular dysfunction after successful reperfusion. Circulation. 2004;109(8):962–5.
van der Horst ICC, Zijlstra F, van’t Hof AWJ, Doggen CJM, de Boer MJ, Suryapranata H, et al. Glucose-insulin-potassium infusion inpatients treated with primary angioplasty for acute myocardial infarction: the glucose-insulin-potassium study a randomized trial. Journal of the American College of Cardiology. 2003 Sep 3;42(5):784–91.
Wargovich TJ, MacDonald RG, Hill JA, Feldman RL, Stacpoole PW, Pepine CJ. Myocardial metabolic and hemodynamic effects of dichloroacetate in coronary artery disease. Am J Cardiology. 1988;61(1):65–70.
Bersin RM, Wolfe C, Kwasman M, Lau D, Klinski C, Tanaka K, et al. Improved hemodynamic function and mechanical efficiency in congestive heart failure with sodium dichloroacetate. J Am Coll Cardiol. 1994;23(7):1617–24.
Chambers KT, Leone TC, Sambandam N, Kovacs A, Wagg CS, Lopaschuk GD, et al. Chronic inhibition of pyruvate dehydrogenase in heart triggers an adaptive metabolic response. J Biol Chem. 2011;286(13):11155–62.
Yan J, Young ME, Cui L, Lopaschuk GD, Liao R, Tian R. Increased glucose uptake and oxidation in mouse hearts prevents high fatty acid oxidation but causes cardiac dysfunction in diet-induced obesity. Circulation. 2009;119(21):2818–28.
El Derh MS, Twab SMA, Elgouhary M. The cardioprotective effect of intralipid in decreasing the ischemic insults during off-pump coronary artery revascularization. Ain-Shams J Anesthesiology. 2021;13(1):61.
Rahman S, Li J, Bopassa JC, Umar S, Iorga A, Partownavid P, et al. Phosphorylation of GSK-3β mediates Intralipid-induced cardioprotection against Ischemia/Reperfusion injury. Anesthesiology. 2011;115(2):242–53.
Watson W, Green P, Rider O, Neubauer S. 81 Increased myocardial fat supply enhances cardiac function in normal hearts and in heart failure. Heart. 2020;106(Suppl 2):A62-3.
Rudolph W, Maas D, Richter J, Hasinger F, Hofmann H, Dohrn P. On the significance of acetoacetate and beta-hydroxybutyrate in human myocardial metabolism. Klin Wochenschr. 1965;15(43):445–51.
Lommi J, Kupari M, Koskinen P, Näveri H, Leinonen H, Pulkki K, et al. Blood ketone bodies in congestive heart failure. J Am College Cardiology. 1996;28(3):665–72.
Bedi KC, Snyder NW, Brandimarto J, Aziz M, Mesaros C, Worth AJ, et al. Evidence for intramyocardial disruption of lipid metabolism and increased myocardial ketone utilization in advanced human heart failure. Circulation. 2016;133(8):706–16.
Schugar RC, Moll AR, André d’Avignon D, Weinheimer CJ, Kovacs A, Crawford PA. Cardiomyocyte-specific deficiency of ketone body metabolism promotes accelerated pathological remodeling. Mol Metab. 2014;3(7):754–69.
Uchihashi M, Hoshino A, Okawa Y, Ariyoshi M, Kaimoto S, Tateishi S, et al. Cardiac-specific Bdh1 overexpression ameliorates oxidative stress and cardiac remodeling in pressure overload–induced heart failure. Circulation Heart Failure. 2017;10(12):e004417.
Gormsen LC, Svart M, Thomsen HH, Søndergaard E, Vendelbo MH, Christensen N, et al. Ketone body infusion with 3-hydroxybutyrate reduces myocardial glucose uptake and increases blood flow in humans: a positron emission tomography study. J Am Heart Assoc. 2017;6(3):e005066.
Renguet E, Ginion A, Gélinas R, Bultot L, Auquier J, Robillard Frayne I, et al. Metabolism and acetylation contribute to leucine-mediated inhibition of cardiac glucose uptake. Am J Physiol Heart Circ Physiol. 2017;313(2):H432-45.
Stanley WC, Meadows SR, Kivilo KM, Roth BA, Lopaschuk GD. beta-Hydroxybutyrate inhibits myocardial fatty acid oxidation in vivo independent of changes in malonyl-CoA content. Am J Physiol Heart Circ Physiol. 2003;285(4):H1626-1631.
Nielsen R, Møller N, Gormsen LC, Tolbod LP, Hansson NH, Sorensen J, et al. Cardiovascular effects of treatment with the ketone body 3-hydroxybutyrate in chronic heart failure patients. Circulation. 2019;139(18):2129–41. This small prospective study on patients with chronic heart failure with reduced ejection fraction (HFrEF) demonstrates the impact of increasing ketone body supply on cardiac haemodynamics and function as well as myocardial efficiency, introducing a potential novel treatment modality in heart failure.
Monzo L, Sedlacek K, Hromanikova K, Tomanova L, Borlaug BA, Jabor A, et al. Myocardial ketone body utilization in patients with heart failure: the impact of oral ketone ester. Metabolism. 2021;115:154452.
Ferrannini E, Mark M, Mayoux E. CV Protection in the EMPA-REG OUTCOME trial: a ‘thrifty substrate’ hypothesis. Diabetes Care. 2016;39(7):1108–14.
Santos-Gallego CG, Requena-Ibanez JA, San Antonio R, Ishikawa K, Watanabe S, Picatoste B, et al. Empagliflozin ameliorates adverse left ventricular remodeling in nondiabetic heart failure by enhancing myocardial energetics. Journal of the American College of Cardiology. 2019;73(15):1931–44. An elegant animal model study on the effect of sodium-glucose cotransporter 2 (SGLT2) inhibition on myocardial substrate metabolism. Using a non-diabetic pig model of ischaemic cardiomyopathy and assessing cardiac function using cardiac MRI and 3-dimensional echocardiography, as well as arterio-venous sampling for metabolite consumption, the authors demonstrate that SGLT2 inhibition enhances LV systolic function, possibly through alteration of myocardial energetics.
Murashige D, Jang C, Neinast M, Edwards JJ, Cowan A, Hyman MC, et al. Comprehensive quantification of fuel use by the failing and nonfailing human heart. Science. 2020;370(6514):364–8.
Hyde R, Taylor PM, Hundal HS. Amino acid transporters: roles in amino acid sensing and signalling in animal cells. Biochem J. 2003;373(Pt 1):1–18.
Sun H, Lu G, Ren S, Chen J, Wang Y. Catabolism of branched-chain amino acids in heart failure: insights from genetic models. Pediatr Cardiol. 2011;32(3):305–10.
Sun H, Olson KC, Gao C, Prosdocimo DA, Zhou M, Wang Z, et al. Catabolic defect of branched-chain amino acids promotes heart failure. Circulation. 2016;133(21):2038–49.
Lai L, Leone TC, Keller MP, Martin OJ, Broman AT, Nigro J, et al. Energy metabolic reprogramming in the hypertrophied and early stage failing heart: a multisystems approach. Circ Heart Fail. 2014;7(6):1022–31.
Neishabouri SH, Hutson SM, Davoodi J. Chronic activation of mTOR complex 1 by branched chain amino acids and organ hypertrophy. Amino Acids. 2015;47(6):1167–82.
Uddin GM, Zhang L, Shah S, Fukushima A, Wagg CS, Gopal K, et al. Impaired branched chain amino acid oxidation contributes to cardiac insulin resistance in heart failure. Cardiovascular Diabetology. 2019;18(1):86.
Uddin GM, Karwi QG, Pherwani S, Gopal K, Wagg CS, Biswas D, et al. Deletion of BCATm increases insulin-stimulated glucose oxidation in the heart. Metabolism. 2021;124:154871.
Chen M, Gao C, Yu J, Ren S, Wang M, Wynn RM, et al. Therapeutic effect of targeting branched-chain amino acid catabolic flux in pressure-overload induced heart failure. J Am Heart Assoc. 2019;8(11):e011625.
Jachthuber Trub C, Balikcioglu M, Freemark M, Bain J, Muehlbauer M, Ilkayeva O, et al. Impact of lifestyle intervention on branched-chain amino acid catabolism and insulin sensitivity in adolescents with obesity. Endocrinol Diabetes Metab. 2021;4(3):e00250.
McGarrah RW, Zhang GF, Christopher BA, Deleye Y, Walejko JM, Page S, et al. Dietary branched-chain amino acid restriction alters fuel selection and reduces triglyceride stores in hearts of Zucker fatty rats. Am J Physiol Endocrinol Metab. 2020;318(2):E216-23.
Sharov VG, Todor AV, Silverman N, Goldstein S, Sabbah HN. Abnormal mitochondrial respiration in failed human myocardium. J Mol Cell Cardiol. 2000;32(12):2361–7.
Sabbah HN, Sharov V, Riddle JM, Kono T, Lesch M, Goldstein S. Mitochondrial abnormalities in myocardium of dogs with chronic heart failure. J Mol Cell Cardiol. 1992;24(11):1333–47.
Dolinsky VW, Cole LK, Sparagna GC, Hatch GM. Cardiac mitochondrial energy metabolism in heart failure role of cardiolipin and sirtuins Biochimica et Biophysica Acta (BBA). Molecular and Cell Biology Lipid. 2016;1861(10):1544–54.
Saini-Chohan HK, Holmes MG, Chicco AJ, Taylor WA, Moore RL, McCune SA, et al. Cardiolipin biosynthesis and remodeling enzymes are altered during development of heart failure. J Lipid Res. 2009;50(8):1600–8.
Dudek J, Hartmann M, Rehling P. The role of mitochondrial cardiolipin in heart function and its implication in cardiac disease. Biochim Biophys Acta Mol Basis Dis. 2019;1865(4):810–21.
Sparagna GC, Chicco AJ, Murphy RC, Bristow MR, Johnson CA, Rees ML, et al. Loss of cardiac tetralinoleoyl cardiolipin in human and experimental heart failure. J Lipid Res. 2007;48(7):1559–70.
Akhmedov AT, Rybin V, Marín-García J. Mitochondrial oxidative metabolism and uncoupling proteins in the failing heart. Heart Fail Rev. 2015;20(2):227–49.
Nickel AG, von Hardenberg A, Hohl M, Löffler JR, Kohlhaas M, Becker J, et al. Reversal of mitochondrial transhydrogenase causes oxidative stress in heart failure. Cell Metab. 2015;22(3):472–84.
Vitamin E. Supplementation and cardiovascular events in high-risk patients. New England J Med. 2000;342(3):154–60.
Adlam VJ, Harrison JC, Porteous CM, James AM, Smith RAJ, Murphy MP, et al. Targeting an antioxidant to mitochondria decreases cardiac ischemia-reperfusion injury. FASEB J. 2005;19(9):1088–95.
Ribeiro Junior RF, Dabkowski ER, Shekar KC. O Connell KA, Hecker PA, Murphy MP MitoQ improves mitochondrial dysfunction in heart failure induced by pressure overload. Free Radic Biol Med. 2018;117:18–29.
Giorgi C, Marchi S, Pinton P. The machineries, regulation and cellular functions of mitochondrial calcium. Nat Rev Mol Cell Biol. 2018;19(11):713–30.
Kirichok Y, Krapivinsky G, Clapham DE. The mitochondrial calcium uniporter is a highly selective ion channel. Nature. 2004;427(6972):360–4.
Belevych AE, Terentyev D, Viatchenko-Karpinski S, Terentyeva R, Sridhar A, Nishijima Y, et al. Redox modification of ryanodine receptors underlies calcium alternans in a canine model of sudden cardiac death. Cardiovasc Res. 2009;84(3):387–95.
Despa S, Islam MA, Weber CR, Pogwizd SM, Bers DM. Intracellular Na+ concentration is elevated in heart failure but Na/K pump function is unchanged. Circulation. 2002;105(21):2543–8.
Hasenfuss G, Reinecke H, Studer R, Meyer M, Pieske B, Holtz J, et al. Relation between myocardial function and expression of sarcoplasmic reticulum Ca(2+)-ATPase in failing and nonfailing human myocardium. Circ Res. 1994;75(3):434–42.
Liu T, Takimoto E, Dimaano VL, DeMazumder D, Kettlewell S, Smith G, et al. Inhibiting mitochondrial Na+/Ca2+ exchange prevents sudden death in a Guinea pig model of heart failure. Circ Res. 2014;115(1):44–54.
Théroux P, Chaitman BR, Danchin N, Erhardt L, Meinertz T, Schroeder JS, et al. Inhibition of the sodium-hydrogen exchanger with cariporide to prevent myocardial infarction in high-risk ischemic situations Main results of the GUARDIAN trial Guard during ischemia against necrosis (GUARDIAN) Investigators. Circulation. 2000;102(25):3032–8.
Mentzer RM, Bartels C, Bolli R, Boyce S, Buckberg GD, Chaitman B, et al. Sodium-hydrogen exchange inhibition by cariporide to reduce the risk of ischemic cardiac events in patients undergoing coronary artery bypass grafting: results of the EXPEDITION study. Ann Thorac Surg. 2008;85(4):1261–70.
Sharov VG, Todor A, Khanal S, Imai M, Sabbah HN. Cyclosporine A attenuates mitochondrial permeability transition and improves mitochondrial respiratory function in cardiomyocytes isolated from dogs with heart failure. J Mol Cell Cardiol. 2007;42(1):150–8.
Ide T, Tsutsui H, Kinugawa S, Utsumi H, Kang D, Hattori N, et al. Mitochondrial electron transport complex I is a potential source of oxygen free radicals in the failing myocardium. Circ Res. 1999;85(4):357–63.
Rosca MG, Vazquez EJ, Kerner J, Parland W, Chandler MP, Stanley W, et al. Cardiac mitochondria in heart failure: decrease in respirasomes and oxidative phosphorylation. Cardiovascular Res. 2008;80(1):30–9.
Mortensen SA, Rosenfeldt F, Kumar A, Dolliner P, Filipiak KJ, Pella D, et al. The effect of coenzyme Q10 on morbidity and mortality in chronic heart failure. JACC Heart Failure. 2014;2(6):641–9.
Wyss M, Kaddurah-Daouk R. Creatine and creatinine metabolism. Physiol Rev. 2000;80(3):1107–213.
Neubauer S, Krahe T, Schindler R, Horn M, Hillenbrand H, Entzeroth C, et al. 31P magnetic resonance spectroscopy in dilated cardiomyopathy and coronary artery disease Altered cardiac high-energy phosphate metabolism in heart failure. Circulation. 1992;86(6):1810–8.
Hansch A, Rzanny R, Heyne JP, Leder U, Reichenbach JR, Kaiser WA. Noninvasive measurements of cardiac high-energy phosphate metabolites in dilated cardiomyopathy by using 31P spectroscopic chemical shift imaging. Eur Radiol. 2005;15(2):319–23.
Beer M, Seyfarth T, Sandstede J, Landschütz W, Lipke C, Köstler H, et al. Absolute concentrations of high-energy phosphate metabolites in normal, hypertrophied, and failing human myocardium measured noninvasively with 31P-SLOOP magnetic resonance spectroscopy. J Am College Cardiol. 2002;40(7):1267–74.
Neubauer S, Remkes H, Spindler M, Horn M, Wiesmann F, Prestle J, et al. Downregulation of the Na+-creatine cotransporter in failing human myocardium and in experimental heart failure. Circulation. 1999;100(18):1847–50.
Nakae I, Mitsunami K, Omura T, Yabe T, Tsutamoto T, Matsuo S, et al. Proton magnetic resonance spectroscopy can detect creatine depletion associated with the progression of heart failure in cardiomyopathy. J Am Coll Cardiol. 2003;42(9):1587–93.
Lygate CA, Aksentijevic D, Dawson D, ten Hove M, Phillips D, de Bono JP, et al. Living without creatine: unchanged exercise capacity and response to chronic myocardial infarction in creatine-deficient mice. Circ Res. 2013;112(6):945–55.
Horn M, Remkes H, Strömer H, Dienesch C, Neubauer S. Chronic phosphocreatine depletion by the creatine analogue β-guanidinopropionate is associated with increased mortality and loss of ATP in rats after myocardial infarction. Circulation. 2001;104(15):1844–9.
Zweier JL, Jacobus WE, Korecky B, Brandejs-Barry Y. Bioenergetic consequences of cardiac phosphocreatine depletion induced by creatine analogue feeding. J Biol Chem. 1991;266(30):20296–304.
ten Hove M, Lygate CA, Fischer A, Schneider JE, Sang AE, Hulbert K, et al. Reduced inotropic reserve and increased susceptibility to cardiac ischemia/reperfusion injury in phosphocreatine-deficient guanidinoacetate-N-methyltransferase-knockout mice. Circulation. 2005;111(19):2477–85.
Lygate CA, Bohl S, ten Hove M, Faller KME, Ostrowski PJ, Zervou S, et al. Moderate elevation of intracellular creatine by targeting the creatine transporter protects mice from acute myocardial infarction. Cardiovasc Res. 2012;96(3):466–75.
Akki A, Su J, Yano T, Gupta A, Wang Y, Leppo MK, et al. Creatine kinase overexpression improves ATP kinetics and contractile function in postischemic myocardium. Am J Physiol Heart Circ Physiol. 2012;303(7):H844-852.
Gupta A, Akki A, Wang Y, Leppo MK, Chacko VP, Foster DB, et al. Creatine kinase–mediated improvement of function in failing mouse hearts provides causal evidence the failing heart is energy starved. J Clin Invest. 2012;122(1):291–302.
Bottomley PA, Panjrath GS, Lai S, Hirsch GA, Wu K, Najjar SS, et al. Metabolic rates of ATP transfer through creatine kinase (CK Flux) predict clinical heart failure events and death. Sci Transl Med. 2013;5(215):2153.
Manchester J, Kong X, Nerbonne J, Lowry OH, Lawrence JC. Glucose transport and phosphorylation in single cardiac myocytes: rate-limiting steps in glucose metabolism. Am J Physiol-Endocrinology Metabol. 1994;266(3):E326-33.
Russell RR, Taegtmeyer H. Pyruvate carboxylation prevents the decline in contractile function of rat hearts oxidizing acetoacetate. Am J Physiol. 1991;261(6 Pt 2):H1756-1762.
Funding
S.M. Ng is supported by the Wellcome Trust Clinical Research Training Fellowship (102176/B/13/Z). O.J. Rider is funded by the British Heart Foundation Senior Clinical Research Fellowship.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ng, S.M., Neubauer, S. & Rider, O.J. Myocardial Metabolism in Heart Failure. Curr Heart Fail Rep 20, 63–75 (2023). https://doi.org/10.1007/s11897-023-00589-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11897-023-00589-y