Abstract
Purpose of Review
This review offers an overview of the evidence in diagnostic and therapeutic applications of remote monitoring implantable devices.
Recent Findings
Remote monitoring of cardiac implantable devices has become more and more popular in recent years as healthcare is moving towards a more patient centralized system. For heart failure patients with an ICD or pacemaker, there is controversial evidence regarding improvements in the clinical outcome, e.g., reduction of hospitalization rates or overall mortality. New developments as hemodynamic remote monitoring via measurement of the pulmonary artery pressure are promising technical achievements showing encouraging results. In cardiac remote monitoring of syncope and arrhythmias, implantable loop recorder plays an important role in diagnostic algorithms.
Summary
Although there is controversial evidence according to remote monitoring of implantable devices, its use is rapidly expanding, giving healthcare providers the opportunity to react promptly to worsening of their patients. Adequate evaluation of the data created by remote monitoring systems remains an unsolved challenge of contemporary healthcare services.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In recent years, especially during the ongoing coronavirus pandemic, remote monitoring (RM) for cardiac implantable electronic devices (CIEDs) has become increasingly popular and accepted. Its use has spread more and more, improving healthcare accessibility, becoming a key factor towards an upcoming more personalized therapy.
For active cardiac devices such as defibrillators (ICDs), pacemakers (PMs), and cardiac resynchronization therapy (CRT), RM is an accessory function that can be activated if necessary. Other devices, such as implantable loop recorders and CardioMEMS™, are set up for the sole purpose of monitoring.
The data obtained from the monitoring system is sent in real time on a database to which the healthcare staff has online access. CIEDs have the ability to send data automatically in cases of device malfunction or detection of life-threatening arrhythmias. Data transmission can also be actively initiated by the patient in case of disturbances or suspicion of worsening of the clinical situation [1,2,3].
This allows avoiding some conventional in-office (IO) follow-up, a condition which is especially advantageous for patients who live far from the referral hospital, as often happens in countries with low distribution of medical centers due to centralization3.
One of the main collectives for which remote monitoring systems are intended is patients with congestive heart failure (CHF), in which the early recognition of a worsening of cardiac performance can allow rapid treatment and thus avoid hospitalization or even death [1,2,3,4].
Although, thanks to advances in medicine, the rate of CHF patients is decreasing, the absolute number of heart failure patients is continuously increasing as the global population and life expectancy. For example, in the UK between 2002 and 2014, CHF incidence decreased by 7%. However, the estimated absolute number of individuals with newly diagnosed CHF increased by 12% [5]. In the USA, the prevalence of CHF will increase 46% from 2012 to 2030, resulting in more than 8 million adult people with CHF [6].
Thus, an ever-wider diffusion of remote monitoring systems is expected in the coming years [7].
The purpose of this article is to give an overview of the most relevant implantable cardiac devices offering RM and the evaluation of their clinical evidence.
Cardiac Monitoring via Pacemakers, Implantable Cardioverter Defibrillators, and Devices for Cardiac Resynchronization Therapy
Currently available hardware devices provided by the different manufacturers are very similar in terms of handling and connectivity (Fig. 1).

© 2022 Abbbott; C LATITUDE™ Home Monitoring System; Boston Scientific. Image provided courtesy of Boston Scientific. ©2022 Boston Scientific Corporation or its affiliates. All rights reserved D Medtronic CareLink™ System, Medtronic
Examples of currently available remote monitoring systems (A CardioMessenger Smart Homemonitoring system, BIOTRONIK SE & Co. KG., Berlin, Germany; B St. Jude Medical, Abbot Merlin@Home™ Transmitter;
Detection of Device-Related Complications
RM in CIEDs is well established in early detection of device-related technical problems and inappropriate shocks [8,9,10,11,12]. In the TRUST trial, ICD patients were randomized to RM with daily transmissions or to conventional care with IO visit only. Lead and generator problems were detected significantly earlier in the RM group compared to the conventional care group (median of 1 vs. 5 days, respectively; p = 0.05) [13]. Furthermore, RM has been demonstrated to be safe and useful in reducing of in-hospital device evaluations [8]. In the study of Watanabe et al., 1274 consecutive patients implanted with a PM have been randomized to RM only or IO follow-up. After 2 years, RM only did not increase the occurrence of death, stroke, or cardiovascular events requiring surgery (10.9% vs. 11.8%, respectively, p < 0.01 for noninferiority), suggesting that RM is not only safe but can be a potential tool to reduce resource consumption as well [14].
Recently, most of the manufacturers of implantable cardiac devices faced problems with lithium plating which randomly can cause rapid battery depletion. Here, activating RM was strictly recommended by the manufacturers and became a beneficial and irreplaceable instrument for both patient and healthcare professional helping to detect affected devices as early as possible [15,16,17].
Detection of Heart Failure Decompensation Using CIEDs
Despite improved outcomes of patients with CHF due to new treatments, morbidity, mortality, and hospitalization rates remain still high [18].
As the 30-day all-cause readmission rate approaches up to 20%, the 10-year mortality reaches almost 100% [19, 20]. Furthermore, hospitalizations account for approximately 70% of the global costs [21].
While optimal medical therapy impacts the natural history of the disease, active cardiac devices like PMs, ICDs, and CRTs have added incremental value in improving heart failure outcomes especially in CHF patients with brady- or tachyarrhythmias [1, 22, 23]. Nevertheless, it has been shown that the conventional clinical practice of routine IO appointments every 6–12 months with additional unscheduled appointments due to worsening of CHF or device malfunctions is ineffective and therefore antiquated [24, 25].
Because CHF decompensation starts weeks before the acute exacerbation, early detection is one of the main goals in RM systems.
RM offers the possibility to assess relevant physiological parameters as heart rate variability (HRV), intrathoracic impedance, and patient activity level to provide information about the clinical status of the patient [26, 27]. It is known that individual parameters for itself have a poor predictive value of CHF decompensation [28]. Modern devices combine several measurements, allowing the implementation of predictive algorithms (e.g., TRIAGE-HF PLUS by Medtronic or HeartLogic by Boston Scientific) to identify patients at increased risk of CHF decompensation [29, 30].
However, inconsistent results in the improvement of RM of CIEDs have been found yet. Several non-randomized trials showed considerable outcome benefits including all-cause mortality [31, 32], whereas most randomized control trials (RCTs) showed neutral effects [8, 33].
In 2015, Parthiban et al. conducted a meta-analysis of nine RCTs, involving 6469 ICD or CRT-D patients, who were randomized to conventional in-office (IO) follow-up and RM [7]. The authors found no significant differences in terms of all-cause mortality (OR 0.83, p = 0.285), CV mortality (OR 0.66, p = 0.103), and hospitalization (OR 0.83, p = 0.196). However, a reduction in all-cause mortality was detected in three trials using RM with daily verification and transmission (OR 0.65, p = 0.021). Of those three investigations, only the IN-TIME trial reported a significant reduction of both, all-cause (HR 0.36, p = 0.004) and CV mortality (HR 0.37, p = 0.012) [34•]. Although in all three studies the probability of receiving any ICD shock was similar in both groups (OR 1.05, p = 0.86), the incidence of inadequate shocks was reduced in RM patients (OR 0.55, p = 0.002) [7].
In 2016, Klersy et al. published a meta-analysis including three additional RCTs, which showed no significant effect of RM on all-cause (relative risk 0.90, p = 0.41) and CV mortality (relative risk 0.93, p = 0.80) or cardiac hospitalization (relative risk 0.96, p = 0.60). However, contrarily to the IN-TIME trial, the authors did not consider the frequency of RM data transmission in their analysis [35].
In the MORE-CARE trial, 865 CRT-D patients were randomized to RM with automated alerts for fluid overload by means of intrathoracic impedance, atrial tachyarrhythmias and system integrity, or IO follow-up alone [36]. The primary endpoint, the composite of death, CV hospitalization, and device-related hospitalization, did not differ significantly between the two randomization groups after 2 years of follow-up (HR 1.02, p = 0.89). Similarly, the individual endpoint components of all-cause mortality (HR 1.13, p = 0.59), CV hospitalization (HR 0.96, p = 0.80), and device-related hospitalization (HR 0.89, p = 0.74) were not different [36].
In the OptiLink-HF trial, 1002 CHF patients with new implanted ICD (37%) or CRT-D (63%) were randomized to RM or to conventional treatment without RM. In the RM group, a daily check of alerts for fluid overload, which led to a specified clinical assessment and treatment, was conducted [37]. No significant difference was found regarding the combined primary endpoint of all-cause death and CV hospitalization (HR 0.87, p = 0.13). The same result was observed concerning both CV mortality and hospitalization due to CHF worsening. Thus, in the OptiLink-HF trial, intrathoracic impedance (IIM) did not significantly improve outcomes in ICD or CRT-D patients with advanced CHF [37].
The poor efficacy of IIM could be explained by some factors, such us transmission failure of RM data, low patient adherence, and insufficient performance of the fluid detection algorithm [2].
Boehmer et al. studied a combination of additional algorithms such as thoracic impedance, heart sounds, relative tidal volume, activity response, heart rate, and respiratory rates. A 70% sensitivity of predicting impending CHF decompensation has been shown combining those indexes [38]. This multiple biosensor algorithm was further evaluated in the MANAGE-HF trial by Hernandez et al. showing promising results in terms of early detection of CHF decompensation [39•].
Other ongoing studies as PREEMPT-HF will add substantial information about the clinical usefulness and effectivity of RM in decision-making compared to conventional care [40].
Detection of Cardiac Arrhythmias
Alongside the detection of life-threatening arrhythmias, one of the main contributions of RM is early detection of atrial arrhythmias (AAR), especially atrial fibrillation (AF), which expose patients to the risk of thromboembolic events, worsening of CHF, inappropriate ICD shocks, or loss of biventricular stimulation [41,42,43].
With RM, physicians might have the opportunity to initiate anticoagulation, where appropriate, and to optimize rate or rhythm control therapies preventing stroke- or AF-related CHF decompensation due to early detection of AF.
In the In-TIME trial, this was discussed to be a key mechanism of benefit where, on subgroup analysis, RM primarily improved outcomes for CHF patients with a history of AF, although this effect was driven by a reduction in all-cause mortality, with no rhythm-specific change in hospitalization [34•].
In the REM-HF trial, Zakeri et al. focused on patients who had device-detected AF in the first year of follow-up. The trial had the goal to evaluate the potential impact of RM on mortality and hospitalization risk by comparing patients with AF to patients in stable sinus rhythm. The use of RM in AF patients was not associated with a reduction in all-cause or cardiovascular mortality. Surprisingly, an increased risk of recurrent cardiovascular and heart failure-related hospitalizations in patients with persistent AF has been observed [44].
Although the authors concluded that RM provided no benefit for patients with CHF compared to usual care, the study showed some interesting issues that might be causative for the neutral outcome. Over one third of the patients transmitted data for less than 75% of the weeks, implying a weak patient adherence. Moreover, centers were overloaded by unfiltered data. For example, approximately only 1% of the data led to a medical attention reflecting the technical and structural limitations of remote monitoring [45].
Although RM theoretically should be more effective in detecting AAR than IO follow-up, due to the continuous rhythm surveillance, concerning data are rather conflicting.
In 2015, Parthiban et al. reported no statistically significant change in the prevalence of AAR detection between RM and IO follow-up (OR 1.24, p = 0.203). However, a significant decrease in the time to event detection and clinical decision was noted with RM compared to IO follow-up (p < 0.001) [7].
In 2017, Amara et al. found similar results in a cohort of 595 pacemaker carriers, which were randomized to activated RM (RM-ON) and disactivated RM (RM-OFF). AAR could be detected in 28% of RM-ON and 22% of RM-OFF group (p = 0.06). Time to AAR detection and treatment was shortened by 44% in the RM-ON group (HR 0.565, p = 0.01). Thus, patients with activated RM were diagnosed and treated earlier for AAR than patients with disactivated RM, experiencing a lower AAR burden [46].
Contrarily, Lima et al. conducted a study randomizing 300 elderly patients after PM implantation to RM and controls. No significance difference in AAR detection was found between the two groups (p = 0.36). Additionally, there was no difference in time to detect the first AF episode in the two groups. However, the median time to detect AF recurrence in the RM group was lower than in the control group (p = 0.004) [47].
A more recent meta-analysis conducted in 16 RCT of patients with CIED or wearable device (WD) reported that RM, compared to IO follow-up, significantly reduced the risk of stroke, which may be due to the reduced time interval between AAR onset and therapeutic intervention [48]. Notably, a higher detection rate of AAR was only noted in patients with wearable devices.
Remote Hemodynamic Monitoring
In the past two decades, different technologies of remote hemodynamic monitoring have been studied. Most evidence is related to the CardioMEMS™ pulmonary artery pressure sensor (Micro-Electro-Mechanical HF System, Abbott Medical, Inc., Abbott Park, IL, USA) which requires no leads or batteries. It is a paper clip-sized device which is directly implanted into the pulmonary artery and concurrently powered and interrogated via an external antenna (Fig. 2). The sensor allows for direct measurements of the pulmonary artery pressure which increase is an early sign of imminent cardiac decompensation [49].
In the CardioMEMS Heart Sensor Allows Monitoring of Pressure to Improve Outcomes in NYHA Class III Heart Failure Patients (CHAMPION) trial, 550 patients with CHF, regardless of left ventricular ejection fraction, were randomized to 2 groups, one in which the clinicians used daily measurement of pulmonal arterial pressure (PAP) in addition to standard of care (treatment group; n 270) versus standard of care alone (control group; n 280) [50••]. The primary endpoint was the rate of heart failure hospitalization over 6 months. There was a significant reduction of the primary endpoint, from a rate of 0.44 in the control group to 0.32 in the treatment group (relative risk reduction, 28%; p 0.0002). Additionally, there was a reduction of 37% in the relative risk of heart failure hospitalizations compared with the control group in the follow-up period (Table 1).
An important subgroup analysis of the CHAMPION trial demonstrated significant efficacy in patients with heart failure and a preserved ejection fraction. The primary efficacy endpoint of heart failure hospitalization rate at 6 months was 46% lower in the treatment group compared with the control group (incidence rate ratio 0.54; p < 0.0001). During follow-up, the hospitalization rate was 50% lower (incidence rate ratio 0.5; p < 0.0001) [51].
Other important subgroups of CHAMPION patients have been analyzed retrospectively. Taken together, these analyses demonstrate the safety and effectiveness of PAP-guided heart failure therapy in patients with a history of myocardial infarction, secondary pulmonary hypertension, and chronic obstructive pulmonary disease and in those with chronic kidney disease [52,53,54].
In the multicenter, single-blinded Guide-HF trial, more than 1000 patients with heart failure NYHA II-IV, irrespective of their ejection fractions, were included. The primary endpoint was a composite of total mortality and total heart failure events. Although PAP-guided therapy management of heart failure did not result in a lower primary endpoint, a pre-COVID-19 analysis indicated a possible benefit in the pre-COVID-19 period primarily driven by lower hospitalizations compared with the control group (HR 0.81, 95% CI 0.66–1.00; p = 0.049) [55].
Whereas most of the studies referring to the CardioMEMS PAP sensor were published in the USA, two European studies in the UK and Germany recently have shown a likely added benefit in NYHA III class patients with a 82% and 62% reduction in CHF annualized hospitalization rates, respectively [56, 57•].
In addition to those remarkable results reflecting the potential of a PAP-guided therapy, several studies showed that CardioMEMS is also effective in terms of comprehensive heart failure cost reduction due to less hospitalization [58, 59].
The ongoing prospective, randomized, open, and multicenter trial PASSPORT-HF examines the efficacy of a pulmonary pressure monitoring in patients with CHF NYHA class III irrespective of ejection fraction and will provide important additional evidence [60].
Implantable Loop Recorder (ILR)
ILR are small subcutaneous implantable devices which provide a long-term continuous ECG monitoring (Fig. 3). Inserted close to the 4th intercostal space, an electrogram, corresponding to lead V2 or V4, is detected by two electrodes integrated at the ends of the devices. Complications of ILR implantations are quite rare, i.e., RIO 2 investigators reported a complication rate of < 1% irrespective of a hospital or ambulatory setting [61, 62]. Currently available ILRs are able to automatically transmit recorded data wirelessly by cell phone or via the provided connectivity hardware to a database of the attending physicians for further evaluation.

© 2022 Abbbott; C Biotronik Biomonitor III™; D Boston Scientific LUX-Dx™. Image provided courtesy of Boston Scientific. ©2022 Boston Scientific Corporation or its affiliates. All rights reserved)
Examples of currently available insertable loop recorders (A Medtronic Linq II™; B St. Jude Medical Confirm Rx™;
Established indications for ILR include unexplained syncope, palpitations, and AF detection [63].
For the diagnostic of palpitation, ILR are indicated in selected cases of severe infrequent symptoms, when other ECG monitoring technologies fail to identify the cause [64].
Most devices are equipped with automated algorithms for the specific recognition of AF, based on R-R wave interval stability parameters or p wave detection failing [65].
Although these algorithms have good sensitivity, the false positive rate is not negligible and requires physicians to personally evaluate the electrograms.
All current guidelines underscore that the decision to implant an ILR should consider patient’s characteristics, frequency of syncope, previous diagnostic work-up, and probability of arrhythmic causes [66,67,68] (Table 2). Evidence for ILR in unexplained syncope has been demonstrated in several RCT and observational studies [69,70,71].
In the setting of AF detection, ILR are generally used to evaluate the efficacy of medical or catheter ablation treatment and to exclude AF as cause of cryptogenic stroke. For detection of subclinical AF after stroke, current guidelines agree on a prolonged ECG monitoring. However, there is no specification of timing and method of monitoring [65].
In the last years, several studies have evidenced a strong association between subclinical AF, especially that of long duration, and increased risk of ischemic stroke. However, a causality could not be proved [65, 72,73,74,75,76,77].
In a multicenter RCT, Svendsen et al. randomized, in a 1:3 ratio, 6004 subjects without a history of AF and at least one risk factor for stroke, to ILR monitoring or usual care. AF was diagnosed significantly more often in the ILR than in the control group (p < 0.0001). However, after initiation of oral anticoagulation therapy in all study subjects, no differences in term of new strokes were detected in the two study groups. The results imply that not all episodes of atrial fibrillation might be associated with a higher risk [78••].
One of the most emblematic RCT for the detection of subclinical AF after cryptogenic stroke or TIA was the Crystal-AF Trial, in which 441 patients were randomized to ILR or standard of care monitoring in a 1:1 ratio [79••]. The primary and secondary endpoints were time to detection of AF, lasting for 30 s or longer at 6 and 12 months, respectively (8.9% vs 1.4% and 12.4% vs 2%). In the ILR arm, the median time to AF detection was 84 days. In a secondary study of Crystal-AF prolonging the follow-up to 3 years, the cumulative AF detection rate was 21.1% at 24 months and 30% at 3 years in the ILR group versus 3% and 3% in the control group. Thus, subclinical AF was detected significantly more often by ILR than by standard of care monitoring. The median time to AF detection was 8.4 months [80].
More recently, Ziegler et al. conducted a prospective observational study to define the incidence of subclinical AF in patients after cryptogenic stroke. Using a database of more than 1200 subjects after ILR implantation and a follow-up of 2 years, the authors reported a median time to AF detection of 112 days [81].
In light of these results, for subclinical AF detection, experts recommend currently a longer monitoring than the 30 days indicated in the past [82, 83]. This is more easily to be made by ILR than by standard of care monitoring using, e.g., Holter, external loop reorders or mobile cardiac telemetry monitors.
All these studies failed to prove a temporal relation between AF detection and further ischemic strokes as well as a clinical benefit of a subsequent oral anticoagulation therapy after AF detection in preventing ischemic events.
Wearable devices (WDs) have recently been shown to be capable of detecting rhythm disturbances, such us AF [84•]. It is therefore plausible to imagine, that in the near future, WD might replace ILRs for some specific indications. However, WDs have not yet been extensively used in clinical routine. Furthermore, no studies have yet been conducted to compare these devices to ILRs.
One aspect to take into consideration is that the increasingly extensive use of simple monitoring systems could lead to a data overload exceeding the assessment capabilities of medical facilities.
Challenges and Future Directions
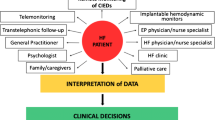
Healthcare is developing towards a more patient-focused model (Fig. 4). Despite the big potential in better addressing healthcare requirements in this context, allowing for early medical response to device alerts RM is still underused in clinical practice [2].
Future developments should focus on technologies which could help to overcome the actual limitations of RM, namely, filtering the large amount of transmitted data to triage patients at risk improving feasibility and efficiency. Artificial intelligence, e.g., which additionally could support diagnostic and treatment decisions including predicting arrhythmias or other cardiovascular diseases, could play a key role in the near future according to that challenge [85, 86]. Supplementary, to streamline the evaluation of RM data, it would be desirable to use a platform capable of integrating data from different manufacturers.
Wearable devices have recently been shown to be able to detect arrhythmias in unselected collectives of the population. Therefore, it is legitimate to think that in the near future, RM could be entrusted to WDs in some if not the majority of patients without an indication for CIEDs. However, data on efficacy of WD in clinical routine is still limited, and further studies are needed in this setting.
Conclusions
The use of remote monitoring in PM, ICD, and CRT devices, complementary to IO follow-ups, has led to an increase in patient satisfaction, an improvement in their quality of life, and greater adherence to the follow-up schedule. Instead, RCTs in this setting demonstrated neutral or discordant results regarding patient outcomes. Other devices with the sole purpose of monitoring, such as ILR and CardioMEMS, certainly provide an improvement in early diagnostics especially in CHF patients.
Daily data transmission seems to be determinant to make RM able to improve patient outcome. However, a large amount of data must be continuously evaluated by the healthcare staff, who must react promptly by offering patients the necessary therapy. Centers able and willing to offer remote monitoring must therefore provide sufficient specialized personnel with the necessary technical equipment that must be updated continuously. Thus, it is of primary importance to carefully select patients, favoring those at risk, such as patients with more depressed LVEF, which are likely to benefit most from RM.
Furthermore, the patient’s potential adherence should be also considered a selection factor for RM, as this is directly correlated with the chances of survival.
The daily review of such amount of RM data could suggest an increase in costs. Conversely, reducing IO follow-up visits and hospitalizations could ultimately lead to a reduction in the overall costs.
Ultimately, future technical developments as integration of artificial intelligence algorithms will provide crucial solutions to overcome current problems in feasibility and efficiency of RM.
Abbreviations
- RM:
-
Remote monitoring
- CIED:
-
Cardiac implantable electronic device
- ICD:
-
Implantable cardiac defibrillator
- PM:
-
Pacemaker
- CRT:
-
Cardiac resynchronization therapy
- IO:
-
In-office
- CHF:
-
Congestive heart failure
- HRV:
-
Heart rate variability
- CV:
-
Cardiovascular
- RCT:
-
Randomized controlled trial
- IIM:
-
Intrathoracic impedance monitoring
- AAR:
-
Atrial arrhythmias
- AF:
-
Atrial fibrillation
- WD:
-
Wearable devices
- PAP:
-
Pulmonal arterial pressure
- ILR:
-
Implantable loop recorder
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
McDonagh TA, Metra M, Adamo M, et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42(36):3599–726. https://doi.org/10.1093/eurheartj/ehab368.
Braunschweig F, Anker SD, Proff J, et al. Remote monitoring of implantable cardioverter-defibrillators and resynchronization devices to improve patient outcomes: dead end or way ahead? Europace. 2019;21:846–55. https://doi.org/10.1093/europace/euz011.
Kelly SE, Campbell D, Duhn LJ, et al. Remote monitoring of cardiovascular implantable electronic devices in Canada: survey of patients and device health care professionals. CJC Open. 2021;3:391e–399. https://doi.org/10.1016/j.cjco.2020.11.010.
Hajduczok AG, Muallem SN, Nudy MS, et al. Remote monitoring for heart failure using implantable devices: a systematic review, meta-analysis, and meta-regression of randomized controlled trials. Heart Fail Rev. 2022;27(4):1281–300. https://doi.org/10.1007/s10741-021-10150-5.
Conrad N, Judge A, Tran J, et al. Temporal trends and patterns in heart failure incidence: a population-based study of 4 million individuals. Lancet. 2018;391(10):572–80. https://doi.org/10.1016/S0140-6736(17)32520-5.
Savarese G, Lund LH. Global public health burden of heart failure. Cardiac Failure Review. 2017;3(1):7–11. https://doi.org/10.15420/cfr.2016:25:2.
Parthiban N, Esterman A, Mahajan R, et al. Remote monitoring of implantable cardioverter-defibrillators: a systematic review and meta-analysis of clinical outcomes. J Am Coll Cardiol. 2015;65:2591–600. https://doi.org/10.1016/j.jacc.2015.04.029.
Varma N, Epstein AE, Irimpen A, et al. Efficacy and safety of automatic remote monitoring for implantable cardioverter-defibrillator follow up: the Lumos-T Safely Reduces Routine Office Device Follow-up (TRUST) trial. Circulation. 2010;122:325–32. https://doi.org/10.1161/CIRCULATIONAHA.110.937409.
Al-Khatib SM, Piccini JP, Knight D, et al. Remote monitoring of implantable cardioverter defibrillators versus quarterly device interrogations in clinic: results from a randomized pilot clinical trial. J Cardiovasc Electrophysiol. 2010;21(5):545–50. https://doi.org/10.1111/j.1540-8167.2009.01659.x.
Guédon-Moreau L, Chevalier P, Marquié C, ECOST trial investigators, et al. Contributions of remote monitoring to the follow-up of implantable cardioverter-defibrillator leads under advisory. Eur Heart J. 2010;31(18):2246–52. https://doi.org/10.1093/eurheartj/ehq203.
Ranasinghe I, Parzynski CS, Freeman JV, et al. Long-term risk for device-related complications and reoperations after implantable cardioverter-defibrillator implantation: an observational cohort study. Ann Intern Med. 2016;165(1):20–9. https://doi.org/10.7326/M15-2732.
Hawkins NM, Grubisic M, Andrade JG, et al. Long-term complications, reoperations and survival following cardioverter-defibrillator implant. Heart. 2018;104(3):237–43. https://doi.org/10.1136/heartjnl-2017-311638.
Varma N, Michalski J, Epstein AE, et al. Automatic remote monitoring of implantable cardioverter-defibrillator lead and generator performance: the Lumos-T Safely RedUceS RouTine Office Device Follow-Up (TRUST) trial. Circ Arrhythm Electrophysiol. 2010;3(5):428–36. https://doi.org/10.1161/CIRCEP.110.951962.
Watanabe E, Yamazaki F, Goto T, et al. Remote management of pacemaker patients with biennial in-clinic evaluation: continuous home monitoring in the Japanese at-home study: a randomized clinical trial. Circ Arrhythm Electrophysiol. 2020;13(5):e007734. https://doi.org/10.1161/CIRCEP.119.007734.
Hayashi Y, Takagi M, Kakihara J, et al. A case of unexpected early battery depletion caused by lithium cluster formation in implantable cardioverter-defibrillator. J Cardiol Cases. 2017;15(6):184–6. https://doi.org/10.1016/j.jccase.2017.02.007.
Aggarwal A, Sarmiento JJ, Charles DR, et al. Accelerated implantable defibrillator battery depletion secondary to lithium cluster formation: a case series. Pacing Clin Electrophysiol. 2016;39(4):375–7. https://doi.org/10.1111/pace.12808.
Pokorney SD, Greenfield RA, Atwater BD, et al. Novel mechanism of premature battery failure due to lithium cluster formation in implantable cardioverter-defibrillators. Heart Rhythm. 2014;11(12):2190–5. https://doi.org/10.1016/j.hrthm.2014.07.038.
Maggioni AP, Dahlström U, Filippatos G, et al. EURObservational Research Programme: regional differences and 1-year follow-up results of the Heart Failure Pilot Survey (ESC-HF Pilot). Eur J Heart Fail. 2013;15(7):808–17. https://doi.org/10.1093/eurjhf/hft050.
Bergethon KE, Ju C, DeVore AD, et al. Trends in 30-day readmission rates for patients hospitalized with heart failure: findings from the get with the guidelines-Heart Failure Registry. Circ Heart Fail. 2016;9(6):10.1161. https://doi.org/10.1161/CIRCHEARTFAILURE.115.002594.
Chun S, Tu JV, Wijeysundera HC, et al. Lifetime analysis of hospitalizations and survival of patients newly admitted with heart failure. Circ Heart Fail. 2012;5(4):414–21. https://doi.org/10.1161/CIRCHEARTFAILURE.111.964791.
Dickstein K, Cohen-Solal A, Filippatos G, et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2008 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association of the ESC (HFA) and endorsed by the European Society of Intensive Care Medicine (ESICM) [published correction appears in Eur Heart J. 2010;12(4):416. https://doi.org/10.1093/eurheartj/ehn309.
Glikson M, Nielsen JC, Kronborg MB, ESC Scientific Document Group, et al. 2021 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy. Eur Heart J. 2021;42(35):3427–520. https://doi.org/10.1093/eurheartj/ehab364.
Heidenreich PA, Bozkurt B, Aguilar D, et al. AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022;145(18):e895–1032. https://doi.org/10.1161/CIR.0000000000001063.
Kotalczyk A, Kalarus Z, Wright DJ, et al. Cardiac electronic devices: future directions and challenges. Med Devices (Auckl). 2020;13:325–38. https://doi.org/10.2147/MDER.S245625.
Imberti JF, Tosetti A, Mei DA, et al. Remote monitoring and telemedicine in heart failure: implementation and benefits. Curr Cardiol Rep. 2021;23(6):55. https://doi.org/10.1007/s11886-021-01487-2.
Yu CM, Wang L, Chau E, et al. Intrathoracic impedance monitoring in patients with heart failure: correlation with fluid status and feasibility of early warning preceding hospitalization. Circulation. 2005;112:841–8. https://doi.org/10.1161/CIRCULATIONAHA.104.492207.
Fang SC, Wu YL, Tsai PS, et al. Heart rate variability and risk of all-cause death and cardiovascular events in patients with cardiovascular disease: a meta-analysis of cohort studies. Biol Res Nurs. 2020;22(1):45–56. https://doi.org/10.1177/1099800419877442.
van Veldhuisen DJ, Braunschweig F, Conraads V, DOT-HF Investigators, et al. Intrathoracic impedance monitoring, audible patient alerts, and outcome in patients with heart failure. Circulation. 2011;124(16):1719–26. https://doi.org/10.1161/CIRCULATIONAHA.111.043042.
Gardner RS, Singh JP, Stancak B, et al. HeartLogic multisensor algorithm identifies patients during periods of significantly increased risk of heart failure events. Circ Hear Fail. 2018;11:e004669. https://doi.org/10.1161/CIRCHEARTFAILURE.117.004669.
Ahmed FZ, Taylor JK, Green C, et al. Triage-HF Plus: a novel device-based remote monitoring pathway to identify worsening heart failure. ESC Hear Fail. 2020;7:108–17. https://doi.org/10.1002/ehf2.12529.
Saxon LA, Hayes DL, Gilliam FR, et al. Long term outcome after ICD and CRT implantation and influence of remote device follow-up: the ALTITUDE survival study. Circulation. 2010;122:2359–67. https://doi.org/10.1161/CIRCULATIONAHA.110.960633.
DeSimone A, Leoni L, Luzi M, et al. Remote monitoring improves outcome after ICD implantation: the clinical efficacy in the management of heart failure (EFFECT) study. Europace. 2015;17:1267–75. https://doi.org/10.1093/europace/euu318.
Guedon-Moreau L, Lacroix D, Sadoul N, et al. A randomized study of remote follow-up of implantable cardioverter defibrillators: safety and efficacy report of the ECOST trial. Eur Heart J. 2013;34:605–14. https://doi.org/10.1093/eurheartj/ehs425.
Hindricks G, Taborsky M, Glikson M, et al. Implant-based multiparameter telemonitoring of patients with heart failure (IN TIME): a randomised controlled trial. Lancet. 2014;384:583–90. https://doi.org/10.1016/S0140-6736(14)61176-4. Important study which showed significant effects of RM on overall mortality.
Klersy C, Boriani G, De Silvestri A, et al. Effect of telemonitoring of cardiac implantable electronic devices on healthcare utilization: a meta-analysis of randomized controlled trials in patients with heart failure. Eur J Heart Fail. 2016;18(2):195–204. https://doi.org/10.1002/ejhf.470.
Boriani G, Da Costa A, Quesada A, et al. Effects of remote monitoring on clinical outcomes and use of healthcare resources in heart failure patients with biventricular defibrillators: results of the MORE-CARE multicentre randomized controlled trial. Eur J Heart Fail. 2017;19:416–25. https://doi.org/10.1002/ejhf.626.
Bohm M, Drexler H, Oswald H, et al. Fluid status telemedicine alerts for heart failure: a randomized controlled trial. Eur Heart J. 2016;37:3154–63. https://doi.org/10.1093/eurheartj/ehw099.
Boehmer JP, Hariharan R, Devecchi FG, et al. A multisensor algorithm predicts heart failure events in patients with implanted devices: results from the MultiSENSE Study. JACC Heart Fail. 2017;5(3):216–25. https://doi.org/10.1016/j.jchf.2016.12.011.
Hernandez AF, Albert NM, Allen LA, et al. MANAGE-HF Study. Multiple cArdiac seNsors for mAnaGEment of Heart Failure (MANAGE-HF) - phase I evaluation of the integration and safety of the HeartLogic multisensor algorithm in patients with heart failure. J Card Fail. 2022:S1071–9164(22)00483–3. https://doi.org/10.1016/j.cardfail.2022.03.349First phase of important trial suggesting safe and successful integration of remote monitoring into clinical care.
Precision event monitoring for patients with heart failure using HeartLogic (PREEMPT-HF) U.S. National Library of Medicine; Bethesda, MD, USA: 2018. NCT03579641.
Bertini M, Marcantoni L, Toselli T, et al. Remote monitoring of implantable device: should we continue to ignore it? Int J Cardiol. 2016;202:368–77. https://doi.org/10.1016/j.ijcard.2015.09.033.
Burri H. Is there a future for remote cardiac implantable electronic device management? Arrhythm Elektrophysiol Rev. 2017;6:109. https://doi.org/10.15420/aer.2017:10:1.
Ono M, Varma N. Remote monitoring for chronic disease management: atrial fibrillation and heart failure. Card Electrophysiol Clin. 2018;10:43–58. https://doi.org/10.1016/j.ccep.2017.11.005.
Zakeri R, Morgan JM, Phillips P, et al. Impact of remote monitoring on clinical outcomes for patients with heart failure and atrial fibrillation: results from the REM-HF trial. Eur J Heart Fail. 2020;22(3):543–53. https://doi.org/10.1002/ejhf.1709.
Morgan JM, Kitt S, Gill J, et al. Remote management of heart failure using implantable electronic devices. Eur Heart J. 2017;38(30):2352–60. https://doi.org/10.1093/eurheartj/ehx227.
Amara W, Montagnier C, Cheggour S, et al. Early detection and treatment of atrial arrhythmias alleviates the arrhythmic burden in paced patients: the SETAM study. Pacing Clin Electrophysiol. 2017;40:527–36. https://doi.org/10.1111/pace.13062.
Lima CEB, Martinelli M, Peixoto GL, et al. Silent atrial fibrillation in elderly pacemaker users: a randomized trail using home monitoring. Ann Noninvasive Electrocardiol. 2016;21:246–55. https://doi.org/10.1111/anec.12294.
Jang JP, Lin HT, Chen YJ, et al. Role of remote monitoring in detection of atrial arrhythmia, stroke reduction, and use of anticoagulant therapy. Circ J. 2020;84:1922–30. https://doi.org/10.1253/circj.CJ-20-0633.
Adamson PB. Pathophysiology of the transition from chronic compensated and acute decompensated heart failure: new insights from continuous monitoring devices. Curr Heart Fail Rep. 2009;6(4):287–92. https://doi.org/10.1007/s11897-009-0039-z.
Abraham WT, Adamson PB, Bourge RC, for the CHAMPION Trial Study Group, et al. Wireless pulmonary artery haemodynamic monitoring in chronic heart failure: a randomised controlled trial. Lancet. 2011;377:658–66. https://doi.org/10.1016/S0140-6736(11)60101-3. Results of the pivotal CHAMPION Trial showing efficacy of remote hemodynamic monitoring to reduce heart failure hospitalizations.
Adamson PB, Abraham WT, Bourge RC, et al. Wireless pulmonary artery pressure monitoring guides management to reduce decompensation in heart failure with preserved ejection fraction. CircHeart Fail. 2014;7:935–44. https://doi.org/10.1016/S0140-6736(11)60101-3.
Krahnke JS, Abraham WT, Adamson PB, Champion Trial Study Group, et al. Heart failure and respiratory hospitalizations are reduced in patients with heart failure and chronic obstructive pulmonary disease with the use of an implantable pulmonary artery pressure monitoring device. J Card Fail. 2015;21:240–9. https://doi.org/10.1016/j.cardfail.2014.12.008.
Shavelle DM, Desai AS, Abraham WT, CardioMEMS Post-Approval Study Investigators, et al. lower rates of heart failure and all-cause hospitalizations during pulmonary artery pressure-guided therapy for ambulatory heart failure: one-year outcomes from the CardioMEMS post-approval study. Circ Heart Fail. 2020;13(8):e006863. https://doi.org/10.1161/CIRCHEARTFAILURE.119.006863.
Abraham WT, Stevenson LW, Bourge RC, et al. Sustained efficacy of pulmonary artery pressure to guide adjustment of chronic heart failure therapy: complete follow-up results from the CHAMPION randomized trial. Lancet. 2016;387:453–61. https://doi.org/10.1016/S0140-6736(15)00723-0.
Lindenfeld J, Zile MR, Desai AS, et al. Haemodynamic-guided management of heart failure (GUIDE-HF): a randomised controlled trial. Lancet. 2021;398(10304):991–1001. https://doi.org/10.1016/S0140-6736(21)01754-2.
Cowie MR, Flett A, Cowburn P, Foley P, Chandrasekaran B, Loke I, et al. Real-world evidence in a national health service: results of the UK CardioMEMS HF System Post-Market Study. ESC Heart Fail. 2022;9(1):48–56. https://doi.org/10.1002/ehf2.13748.
Angermann CE, Assmus B, Anker SD, et al. Pulmonary artery pressure-guided therapy in ambulatory patients with symptomatic heart failure: the CardioMEMS European monitoring study for heart failure (MEMS-HF). Eur J Heart Fail. 2020;22:1891–901. https://doi.org/10.1002/ejhf.1943. First study to suggest generalisability of US CardioMEMS findings to rest of western healthcare system.
Desai AS, Bhimaraj A, Bharmi R, et al. Ambulatory hemodynamic monitoring reduces heart failure hospitalizations in “real-world” clinical practice. J Am Coll Cardiol. 2017;69:2357–65. https://doi.org/10.1016/j.jacc.2017.03.009.
Martinson M, Bharmi R, Dalal N, et al. Pulmonary artery pressure-guided heart failure management: US cost-effectiveness analyses using the results of the CHAMPION clinical trial. Eur J Heart Fail. 2017;19:652–60. https://doi.org/10.1002/ejhf.642.
Störk S, Bernhardt A, Böhm M, et al. Pulmonary artery sensor system pressure monitoring to improve heart failure outcomes (PASSPORT-HF): rationale and design of the PASSPORT-HF multicenter randomized clinical trial. Clin Res Cardiol. 2022;4:1–11. https://doi.org/10.1007/s00392-022-01987-3.
Pachulsky R, Cockrell J, Salomon H, et al. Implant evaluation of an insertable cardiac monitor outside the electrophysiology labsetting. PloSOne. 2013;8(8):e71544. https://doi.org/10.1371/journal.pone.0071544.
Rogers JD, Sanders P, Piorkowski C, et al. In-office insertion of a miniaturized insertable cardiac monitor: results from the Reveal LINQ In-Office 2 randomized study. Heart Rhythm. 2017;14(2):218–24. https://doi.org/10.1016/j.hrthm.2016.11.001.
Giada F, Bertraglia E, Reimers B, et al. Current and emerging indications for implantable cardiac monitors. Pacing Clin Electrophysiol. 2012;35:1169–78. https://doi.org/10.1111/j.1540-8159.2012.03411.x.
Brignole M, Vardas P, Hoffmann E, et al. Indications for the use of diagnostic implantable and external ECG loop recorders. Europace. 2009;11:67–87. https://doi.org/10.1093/europace/eup097.
Giancaterino S, Lupercio F, Nishimura M, et al. Current and future of insertable cardiac monitors. JACC Clin Electrophysiol. 2018;4(11):1383–96. https://doi.org/10.1016/j.jacep.2018.06.001.
Brignole M, Moya A, de Lange FJ, et al. ESC Guidelines for the diagnosis and management of syncope. Eur Heart J. 2018;39(21):1883–948. https://doi.org/10.1093/eurheartj/ehy037.
Shen WK, Sheldon RS, Benditt DG, et al. 2017 ACC/AHA/HRS guideline for the evaluation and management of patients with syncope: a report of the American College of Cardiology/American Heart association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2017;70(5):e39–110. https://doi.org/10.1016/j.jacc.2017.03.003.
Steinberg JS, Varna N, Cygankiewicz I, et al. 2017 ISHNE-HRS expert consensus statement on ambulatory ECG and external cardiac monitoring/telemetry. Heart Rhythm. 2017;14:e55-96. https://doi.org/10.1016/j.hrthm.2017.03.038.
Krahn AD, Klein GJ, Yee R, et al. Randomized assessment of syncope trial: conventional diagnostic versus a prolonged monitoring strategy. Circulation. 2001;104:46–51. https://doi.org/10.1161/01.cir.104.1.46.
Edvardsson N, Frykman V, van Mechelen R, et al. Use of an implantable loop recorder to increase the diagnostic yield in unexplained syncope: results from the PICTURE registry. Europace. 2011;13:262–9. https://doi.org/10.1093/europace/euq418.
Solbiati M, Costantino G, Casazza G, et al. Implantable loop recorder versus conventional diagnostic workup for unexplained recurrent syncope. Cochrane Database Syst Rev. 2016;4:CD011637. https://doi.org/10.1002/14651858.CD011637.pub2.
Healey JS, Connolly SJ, Gold MR, et al. Subclinical atrial fibrillation and the risk of stroke. N Engl J Med. 2012;366:120–9. https://doi.org/10.1056/NEJMoa1105575.
Verma A, Cairns JA, Mitchell LB, et al. 2014 Focused update of the Canadian Cardiovascular Society guidelines for the management of atrial fibrillation. Can J Cardiol. 2014;30:1114–30. https://doi.org/10.1016/j.cjca.2014.08.001.
Glotzer TV, Daoud EG, Wyse DG, et al. The relationship between daily atrial tachyarrhythmias burden from implantable device diagnostics and stroke risk: the TRENDS study. Circ Arrhythm Electrophysiol. 2009;2:474–80. https://doi.org/10.1161/CIRCEP.109.849638.
Glotzer TV, Hellkamp AS, Zimmermann J, et al. Atrial high rate episodes detected by pacemaker diagnostics predict death and stroke: report of the Atrial Diagnostics Ancillary Study of the Mode Selection Trial (MOST). Circulation. 2003;107:1614–9. https://doi.org/10.1161/01.CIR.0000057981.70380.45.
Van Gelder IC, Healey JS, Crijns HJGM, et al. Duration of device-detected subclinical atrial fibrillation and occurrence of stroke in ASSERT. Eur Heart J. 2017;38:1339–44. https://doi.org/10.1093/eurheartj/ehx042.
Mahajan R, Perera T, Elliott AD, et al. Subclinical device-detected atrial fibrillation and stroke risk: a systematic review and meta-analysis. Eur Heart J. 2018;39:1409–15. https://doi.org/10.1093/eurheartj/ehx731.
Svendsen JH, Diederichsen SZ, Højberg S, et al. Implantable loop recorder detection of atrial fibrillation to prevent stroke (The LOOP Study): a randomised controlled trial. Lancet. 2021;398(10310):1507–16. https://doi.org/10.1016/S0140-6736(21)01698-6. This study was conducted in a large population comparing ILR to usual care diagnostic for detection of AF in patients at risk of stroke.
Sanna T, Diener HC, Passman RS, et al. Cryptogenic stroke and underlying atrial fibrillation. N Egl J Med. 2014;370:2478–86. https://doi.org/10.1056/NEJMoa1313600. One of the most important RCT for the detection of subclinical AF after cryptogenic stroke or TIA, randomizing ILR and standard of care monitoring.
Brachmann J, Morillo CA, Sanna T, et al. Uncovering atrial fibrillation beyon short-term monitoring in cryptogenic stroke patients: three-years results from the Cryptogenic Stroke and Underlying Atrial Fibrillation Trial. Circ Arrhythm Electrophysiol. 2016;9:e003333. https://doi.org/10.1161/CIRCEP.115.003333.
Ziegler PD, Rogers JD, Fereira SW, et al. Long-term detection of atrial fibrillation with insertable cardiac monitors in a real-world cryptogenic stroke population. Int J Cardiol. 2017;244:175–9. https://doi.org/10.1016/j.ijcard.2017.06.039.
Albers GW, Bernstein RA, Brachmann J, et al. Heart rhythm monitoring strategies of cryptogenic stroke: 2015 Diagnostics and Monitoring Stroke Focus Group Report. J Am Heaert Assoc. 2016;5:e002944. https://doi.org/10.1161/JAHA.115.002944.
Kernan WN, Ovbiagele B, Black HR, et al. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45:2160–236. https://doi.org/10.1161/STR.0000000000000024.
Perez MV, Mahaffey KW, Hedlin H, et al. Large-scale of smartwatch to identify atrial fibrillation. N Engl J Med. 2019;381:1909–17. https://doi.org/10.1056/NEJMoa1901183. One of the most important trails of wearables for detecting atrial fibrillation.
Pevnick JM, Birkeland K, Zimmer R, et al. Wearable technology for cardiology: an update and framework for the future. Trends Cardiovasc Med. 2018;28:144–50. https://doi.org/10.1016/j.tcm.2017.08.003.
Silva-Cardoso J, Juanatey JRG, Comin-Colet J, et al. The future of telemedicine in the management of heart failure patients. Card Fail Rev. 2021;7:e11. https://doi.org/10.15420/cfr.2020.32.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Devices
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Reinhardt, A., Ventura, R. Remote Monitoring of Cardiac Implantable Electronic Devices: What is the Evidence?. Curr Heart Fail Rep 20, 12–23 (2023). https://doi.org/10.1007/s11897-023-00586-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11897-023-00586-1