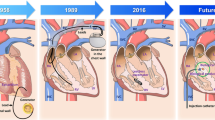
Abstract
Purpose of Review
The goal of this paper is to review present knowledge regarding biological pacemakers created by somatic reprogramming as a platform for mechanistic and metabolic understanding of the rare subpopulation of pacemaker cells, with the ultimate goal of creating biological alternatives to electronic pacing devices.
Recent Findings
Somatic reprogramming of cardiomyocytes by reexpression of embryonic transcription factor T-box 18 (TBX18) converts them into pacemaker-like. Recent studies take advantage of this model to gain insight into the electromechanical, metabolic, and architectural intricacies of the cardiac pacemaker cell across various models, including a surgical model of complete atrioventricular block (CAVB) in adult rats.
Summary
The studies reviewed here reinforce the potential utility of TBX18-induced pacemaker myocytes (iPMS) as a minimally invasive treatment for heart block. Several challenges which must be overcome to develop a viable therapeutic intervention based on these observations are discussed.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Bleeker WK, Mackaay AJ, Masson-Pevet M, Bouman LN, Becker AE. Functional and morphological organization of the rabbit sinus node. Circ Res. 1980;46(1):11–22. https://doi.org/10.1161/01.res.46.1.11.
Cho HC, Marban E. Biological therapies for cardiac arrhythmias: can genes and cells replace drugs and devices? Circ Res. 2010;106(4):674–85. https://doi.org/10.1161/circresaha.109.212936.
Tracy CM, Epstein AE, Darbar D, DiMarco JP, Dunbar SB, Estes NA 3rd, et al. 2012 ACCF/AHA/HRS focused update of the 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Thorac Cardiovasc Surg. 2012;144(6):e127–45. https://doi.org/10.1016/j.jtcvs.2012.08.032.
Bordachar P, Zachary W, Ploux S, Labrousse L, Haissaguerre M, Thambo J-B. Pathophysiology, clinical course, and management of congenital complete atrioventricular block. Heart Rhythm. 2013;10(5):760–6. https://doi.org/10.1016/j.hrthm.2012.12.030.
Cho HC. Pacing the heart with genes: recent progress in biological pacing. Curr Cardiol Rep. 2015;17(8):65. https://doi.org/10.1007/s11886-015-0620-x.
Kapoor N, Galang G, Marban E, Cho HC. Transcriptional suppression of connexin43 by TBX18 undermines cell-cell electrical coupling in postnatal cardiomyocytes. J Biol Chem. 2011;286(16):14073–9. https://doi.org/10.1074/jbc.M110.185298.
Kapoor N, Liang W, Marban E, Cho HC. Direct conversion of quiescent cardiomyocytes to pacemaker cells by expression of Tbx18. Nat Biotechnol. 2013;31(1):54–62. https://doi.org/10.1038/nbt.2465.
Hu Y-F, Dawkins JF, Cho HC, Marbán E, Cingolani E. Biological pacemaker created by minimally invasive somatic reprogramming in pigs with complete heart block. Sci Transl Med. 2014;6(245):245ra94. https://doi.org/10.1126/scitranslmed.3008681.
Oros A, Beekman JDM, Vos MA. The canine model with chronic, complete atrio-ventricular block. Pharmacol Ther. 2008;119(2):168–78. https://doi.org/10.1016/j.pharmthera.2008.03.006.
Piron J, Quang KL, Briec F, Amirault J-C, Leoni A-L, Desigaux L, et al. Biological pacemaker engineered by nonviral gene transfer in a mouse model of complete atrioventricular block. Mol Ther. 2008;16(12):1937–43. https://doi.org/10.1038/mt.2008.209.
Le Quang K, Benito B, Naud P, Qi Xiao Y, Shi Yan F, Tardif J-C, et al. T-type calcium current contributes to escape automaticity and governs the occurrence of lethal arrhythmias after atrioventricular block in mice. Circ Arrhythm Electrophysiol. 2013;6(4):799–808. https://doi.org/10.1161/CIRCEP.113.000407.
Park J, Ryu J, Choi SK, Seo E, Cha JM, Ryu S, et al. Real-time measurement of the contractile forces of self-organized cardiomyocytes on hybrid biopolymer microcantilevers. Anal Chem. 2005;77(20):6571–80. https://doi.org/10.1021/ac0507800.
Plonsey R, Barr RC. Bioelectricity: a quantitative approach. Boston: Springer; 2007.
Tung L, Sliz N, Mulligan MR. Influence of electrical axis of stimulation on excitation of cardiac muscle cells. Circ Res. 1991;69(3):722–30. https://doi.org/10.1161/01.RES.69.3.722.
• Sayegh MN, Fernandez N, Cho HC. Strength-duration relationship as a tool to prioritize cardiac tissue properties that govern electrical excitability. Am J Physiol Heart Circ Physiol. 2019;317(1):H13–25. https://doi.org/10.1152/ajpheart.00161.2019This experiment defined the strength-duration relationship as a metric of pacemaker function.
Radisic M, Park H, Shing H, Consi T, Schoen FJ, Langer R, et al. Functional assembly of engineered myocardium by electrical stimulation of cardiac myocytes cultured on scaffolds. Proc Natl Acad Sci. 2004;101(52):18129–34. https://doi.org/10.1073/pnas.0407817101.
Kohlhardt M, Mnich Z, Maier G. Alterations of the excitation process of the sinoatrial pacemaker cell in the presence of anoxia and metabolic inhibitors. J Mol Cell Cardiol. 1977;9(6):477–88. https://doi.org/10.1016/S0022-2828(77)80027-8.
Nishi K, Yoshikawa Y, Sugahara K, Morioka T. Changes in electrical activity and ultrastructure of sinoatrial nodal cells of the rabbit's heart exposed to hypoxic solution. Circ Res. 1980;46(2):201–13. https://doi.org/10.1161/01.RES.46.2.201.
• Gu J-M, Grijalva SI, Fernandez N, Kim E, Foster DB, Cho HC. Induced cardiac pacemaker cells survive metabolic stress owing to their low metabolic demand. Exp Mol Med. 2019;51(9):105. https://doi.org/10.1038/s12276-019-0303-6These experiments defined the metabolic profile of induced pacemaker cells.
Parra V, Verdejo H, del Campo A, Pennanen C, Kuzmicic J, Iglewski M, et al. The complex interplay between mitochondrial dynamics and cardiac metabolism. J Bioenerg Biomembr. 2011;43(1):47–51. https://doi.org/10.1007/s10863-011-9332-0.
Youle RJ, van der Bliek AM. Mitochondrial fission, fusion, and stress. Science. 2012;337(6098):1062–5. https://doi.org/10.1126/science.1219855.
Cogliati S, Enriquez JA, Scorrano L. Mitochondrial cristae: where beauty meets functionality. Trends Biochem Sci. 2016;41(3):261–73. https://doi.org/10.1016/j.tibs.2016.01.001.
Gottlieb RA, Bernstein D. Mitochondrial remodeling: rearranging, recycling, and reprogramming. Cell Calcium. 2016;60(2):88–101. https://doi.org/10.1016/j.ceca.2016.04.006.
Anumonwo JMB, Lopatin AN. Cardiac strong inward rectifier potassium channels. J Mol Cell Cardiol. 2010;48(1):45–54. https://doi.org/10.1016/j.yjmcc.2009.08.013.
Unudurthi SD, Wolf RM, Hund TJ. Role of sinoatrial node architecture in maintaining a balanced source-sink relationship and synchronous cardiac pacemaking. Front Physiol. 2014;5:446. https://doi.org/10.3389/fphys.2014.00446.
Noble D. A modification of the Hodgkin—Huxley equations applicable to Purkinje fibre action and pacemaker potentials. J Physiol. 1962;160(2):317–52. https://doi.org/10.1113/jphysiol.1962.sp006849.
Trenor B, Cardona K, Saiz J, Noble D, Giles W. Cardiac action potential repolarization revisited: early repolarization shows all-or-none behaviour. J Physiol. 2017;595(21):6599–612. https://doi.org/10.1113/JP273651.
Joyner RW, Wilders R, Wagner MB. Propagation of pacemaker activity. Med Biol Eng Comput. 2007;45(2):177–87. https://doi.org/10.1007/s11517-006-0102-9.
Rohr S, Kucera JP, Fast VG, Kléber AG. Paradoxical improvement of impulse conduction in cardiac tissue by partial cellular uncoupling. Science. 1997;275(5301):841–4.
• Grijalva SI, Gu J-M, Li J, Fernandez N, Fan J, Sung Jung H, et al. Engineered cardiac pacemaker nodes created by TBX18 gene transfer overcome source-sink mismatch. Adv Sci (Weinh). 2019;6(22):1901099. https://doi.org/10.1002/advs.201901099This paper presented constructs that recapituate the in silico parameters needed to overcome source-sink mismatch in a tissue model.
Bouman LN, Duivenvoorden JJ, Bukauskas FF, Jongsma HJ. Anisotropy of electrotonus in the sinoatrial node of the rabbit heart. J Mol Cell Cardiol. 1989;21(4):407–18. https://doi.org/10.1016/0022-2828(89)90651-2.
Cingolani E, Yee K, Shehata M, Chugh SS, Marbán E, Cho HC. Biological pacemaker created by percutaneous gene delivery via venous catheters in a porcine model of complete heart block. Heart Rhythm. 2012;9(8):1310–8. https://doi.org/10.1016/j.hrthm.2012.04.020.
Dawkins JF, Hu Y-F, Valle J, Sanchez L, Zheng Y, Marbán E, et al. Antegrade conduction rescues right ventricular pacing-induced cardiomyopathy in complete heart block. J Am Coll Cardiol. 2019;73(13):1673–87. https://doi.org/10.1016/j.jacc.2018.12.086.
Protze SI, Liu J, Nussinovitch U, Ohana L, Backx PH, Gepstein L, et al. Sinoatrial node cardiomyocytes derived from human pluripotent cells function as a biological pacemaker. Nat Biotechnol. 2016;35:56. https://doi.org/10.1038/nbt.3745 https://www.nature.com/articles/nbt.3745#supplementary-information. Accessed 12 Dec 2016
Boink GJJ, Robinson RB. Gene therapy for restoring heart rhythm. J Cardiovasc Pharmacol Ther. 2014;19(5):426–38. https://doi.org/10.1177/1074248414528575.
• Kim NK, Wolfson D, Fernandez N, Shin M, Cho HC. A rat model of complete atrioventricular block recapitulates clinical indices of bradycardia and provides a platform to test disease-modifying therapies. Sci Rep. 2019;9(1):6930. https://doi.org/10.1038/s41598-019-43300-9The paper was built upon previous in silico, in vitro, and animal models and established a rodent CAVD model.
Bignolais O, Quang KL, Naud P, El Harchi A, Briec F, Piron J, et al. Early ion-channel remodeling and arrhythmias precede hypertrophy in a mouse model of complete atrioventricular block. J Mol Cell Cardiol. 2011;51(5):713–21. https://doi.org/10.1016/j.yjmcc.2011.07.008.
Bernstein BS, Silver ES, Liberman L. QT prolongation and torsades de pointes in a patient with heart block and a pacemaker. Cardiol Young. 2016;26(1):161–3. https://doi.org/10.1017/S1047951114002674.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Angel Xiao declares no conflict of interest. Hee Cheol Cho has a patent US Patent Number 14/357,195 issued.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Regenerative Medicine
Rights and permissions
About this article
Cite this article
Xiao, A., Cho, H.C. Cellular Reprogramming Approaches to Engineer Cardiac Pacemakers. Curr Cardiol Rep 22, 29 (2020). https://doi.org/10.1007/s11886-020-01281-6
Published:
DOI: https://doi.org/10.1007/s11886-020-01281-6