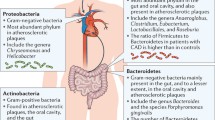
Abstract
Purpose of Review
Studies in microbiota-mediated health risks have gained traction in recent years since the compilation of the Human Microbiome Project. No longer do we believe that our gut microbiota is an inert set of microorganisms that reside in the body without consequence. In this review, we discuss the recent findings which further our understanding of the connection between the gut microbiota and the atherosclerosis.
Recent Findings
We evaluate studies which illustrate the current understanding of the relationship between infection, immunity, altered metabolism, and bacterial products such as immune activators or dietary metabolites and their contributions to the development of atherosclerosis. In particular, we critically examine rec ent clinical and mechanistic findings for the novel microbiota-dependent dietary metabolite, trimethylamine N-oxide (TMAO), which has been implicated in atherosclerosis. These discoveries are now becoming integrated with advances in microbiota profiling which enhance our ability to interrogate the functional role of the gut microbiome and develop strategies for targeted therapeutics.
Summary
The gut microbiota is a multi-faceted system that is unraveling novel contributors to the development and progression of atherosclerosis. In this review, we discuss historic and novel contributors while highlighting the TMAO story mainly as an example of the various paths taken beyond deciphering microbial composition to elucidate downstream mechanisms that promote (or protect from) atherogenesis in the hopes of translating these findings from bench to bedside.
Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Gilbert A, Lion G. Arterites infectieuses experimentales. Comptes Rendus Hebdomadaires des Seances et Memoires de la Societe de Biologie. 1889;41:583–4.
Zhang T, Kurita-Ochiai T, Hashizume T, Du Y, Oguchi S, Yamamoto M. Aggregatibacter actinomycetemcomitans accelerates atherosclerosis with an increase in atherogenic factors in spontaneously hyperlipidemic mice. FEMS Immunol Med Microbiol. 2010;59(2):143–51.
Hayashi C, Viereck J, Hua N, et al. Porphyromonas gingivalis accelerates inflammatory atherosclerosis in the innominate artery of ApoE deficient mice. Atherosclerosis. 2011;215(1):52–9.
Hayashi C, Madrigal AG, Liu X, et al. Pathogen-mediated inflammatory atherosclerosis is mediated in part via toll-like receptor 2-induced inflammatory responses. J Innate Immun. 2010;2(4):334–43.
Mattila KJ, Nieminen MS, Valtonen VV, et al. Association between dental health and acute myocardial infarction. BMJ. 1989;298(6676):779–81.
Kalayoglu MV, Byrne GI. Induction of macrophage foam cell formation by Chlamydia pneumoniae. J Infect Dis. 1998;177(3):725–9.
Coombes BK, Mahony JB. Chlamydia pneumoniae infection of human endothelial cells induces proliferation of smooth muscle cells via an endothelial cell-derived soluble factor(s). Infect Immun. 1999;67(6):2909–15.
Blessing E, Campbell LA, Rosenfeld ME, Chough N, Kuo CC. Chlamydia pneumoniae infection accelerates hyperlipidemia induced atherosclerotic lesion development in C57BL/6J mice. Atherosclerosis. 2001;158(1):13–7.
Saikku P, Leinonen M, Tenkanen L, et al. Chronic Chlamydia pneumoniae infection as a risk factor for coronary heart disease in the Helsinki Heart Study. Ann Intern Med. 1992;116(4):273–8.
Calandrini CA, Ribeiro AC, Gonnelli AC, et al. Microbial composition of atherosclerotic plaques. Oral Dis. 2014;20(3):e128–34.
Rosenfeld ME, Campbell LA. Pathogens and atherosclerosis: update on the potential contribution of multiple infectious organisms to the pathogenesis of atherosclerosis. Thromb Haemost. 2011;106(5):858–67.
Hayashi C, Gudino CV, Gibson FC 3rd, Genco CA. Review: pathogen-induced inflammation at sites distant from oral infection: bacterial persistence and induction of cell-specific innate immune inflammatory pathways. Mol Oral Microbiol. 2010;25(5):305–16.
Hogdahl M, Soderlund G, Kihlstrom E. Expression of chemokines and adhesion molecules in human coronary artery endothelial cells infected with Chlamydia (Chlamydophila) pneumoniae. APMIS. 2008;116(12):1082–8.
Wright SD, Burton C, Hernandez M, et al. Infectious agents are not necessary for murine atherogenesis. J Exp Med. 2000;191(8):1437–42.
Stepankova R, Tonar Z, Bartova J, et al. Absence of microbiota (germ-free conditions) accelerates the atherosclerosis in ApoE-deficient mice fed standard low cholesterol diet. J Atheroscler Thromb. 2010;17(8):796–804.
Saita D, Ferrarese R, Foglieni C, et al. Adaptive immunity against gut microbiota enhances apoE-mediated immune regulation and reduces atherosclerosis and western-diet-related inflammation. Sci Rep. 2016;6:29353.
Jespersen CM, Als-Nielsen B, Damgaard M, et al. Randomised placebo controlled multicentre trial to assess short term clarithromycin for patients with stable coronary heart disease: CLARICOR trial. BMJ. 2006;332(7532):22–7.
Cannon CP, Braunwald E, McCabe CH, et al. Antibiotic treatment of Chlamydia pneumoniae after acute coronary syndrome. N Engl J Med. 2005;352(16):1646–54.
Grayston JT, Kronmal RA, Jackson LA, et al. Azithromycin for the secondary prevention of coronary events. N Engl J Med. 2005;352(16):1637–45.
O’Connor CM, Dunne MW, Pfeffer MA, et al. Azithromycin for the secondary prevention of coronary heart disease events: the WIZARD study: a randomized controlled trial. JAMA. 2003;290(11):1459–66.
Ding Y, Subramanian S, Montes VN, et al. Toll-like receptor 4 deficiency decreases atherosclerosis but does not protect against inflammation in obese low-density lipoprotein receptor-deficient mice. Arterioscler Thromb Vasc Biol. 2012;32(7):1596–604.
Jin C, Henao-Mejia J, Flavell RA. Innate immune receptors: key regulators of metabolic disease progression. Cell Metab. 2013;17(6):873–82.
Michelsen KS, Wong MH, Shah PK, et al. Lack of toll-like receptor 4 or myeloid differentiation factor 88 reduces atherosclerosis and alters plaque phenotype in mice deficient in apolipoprotein E. Proc Natl Acad Sci U S A. 2004;101(29):10679–84.
Higashimori M, Tatro JB, Moore KJ, Mendelsohn ME, Galper JB, Beasley D. Role of toll-like receptor 4 in intimal foam cell accumulation in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2011;31(1):50–7.
Naiki Y, Sorrentino R, Wong MH, et al. TLR/MyD88 and liver X receptor alpha signaling pathways reciprocally control Chlamydia pneumoniae-induced acceleration of atherosclerosis. J Immunol. 2008;181(10):7176–85.
Hayashi C, Papadopoulos G, Gudino CV, et al. Protective role for TLR4 signaling in atherosclerosis progression as revealed by infection with a common oral pathogen. J Immunol. 2012;189(7):3681–8.
Duewell P, Kono H, Rayner KJ, et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 2010;464(7293):1357–61.
Ridker PM, Thuren T, Zalewski A, Libby P. Interleukin-1beta inhibition and the prevention of recurrent cardiovascular events: rationale and design of the Canakinumab Anti-inflammatory Thrombosis Outcomes Study (CANTOS). Am Heart J. 2011;162(4):597–605.
Sayin SI, Wahlstrom A, Felin J, et al. Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-beta-muricholic acid, a naturally occurring FXR antagonist. Cell Metab. 2013;17(2):225–35.
Li T, Chiang JY. Bile acid signaling in metabolic disease and drug therapy. Pharmacol Rev. 2014;66(4):948–83.
Li T, Chiang JY. Bile acids as metabolic regulators. Curr Opin Gastroenterol. 2015;31(2):159–65.
Calkin AC, Tontonoz P. Transcriptional integration of metabolism by the nuclear sterol-activated receptors LXR and FXR. Nat Rev Mol Cell Biol. 2012;13(4):213–24.
Hanniman EA, Lambert G, McCarthy TC, Sinal CJ. Loss of functional farnesoid X receptor increases atherosclerotic lesions in apolipoprotein E-deficient mice. J Lipid Res. 2005;46(12):2595–604.
Bishop-Bailey D, Walsh DT, Warner TD. Expression and activation of the farnesoid X receptor in the vasculature. Proc Natl Acad Sci U S A. 2004;101(10):3668–73.
Zhang Y, Wang X, Vales C, et al. FXR deficiency causes reduced atherosclerosis in Ldlr−/− mice. Arterioscler Thromb Vasc Biol. 2006;26(10):2316–21.
Kim I, Ahn SH, Inagaki T, et al. Differential regulation of bile acid homeostasis by the farnesoid X receptor in liver and intestine. J Lipid Res. 2007;48(12):2664–72.
Ley RE, Lozupone CA, Hamady M, Knight R, Gordon JI. Worlds within worlds: evolution of the vertebrate gut microbiota. Nat Rev Microbiol. 2008;6(10):776–88.
Xue Z, Zhang W, Wang L, et al. The bamboo-eating giant panda harbors a carnivore-like gut microbiota, with excessive seasonal variations. MBio. 2015;6(3):e00022–15.
Goodrich JK, Waters JL, Poole AC, et al. Human genetics shape the gut microbiome. Cell. 2014;159(4):789–99.
Ubeda C, Lipuma L, Gobourne A, et al. Familial transmission rather than defective innate immunity shapes the distinct intestinal microbiota of TLR-deficient mice. J Exp Med. 2012;209(8):1445–56.
Org E, Parks BW, Joo JW, et al. Genetic and environmental control of host-gut microbiota interactions. Genome Res. 2015;25(10):1558–69.
Yancey PH, Gerringer ME, Drazen JC, Rowden AA, Jamieson A. Marine fish may be biochemically constrained from inhabiting the deepest ocean depths. Proc Natl Acad Sci U S A. 2014;111(12):4461–5.
Bennion BJ, Daggett V. Counteraction of urea-induced protein denaturation by trimethylamine N-oxide: a chemical chaperone at atomic resolution. Proc Natl Acad Sci U S A. 2004;101(17):6433–8.
•• Wang Z, Klipfell E, Bennett BJ, et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472(7341):57–63. Seminal study which first linked microbiota dependent metabolite, TMAO, with atherosclerosis.
Koeth RA, Wang Z, Levison BS, et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med. 2013;19(5):576–85.
Ma G, Pan B, Chen Y, Guo C, Zhao M, Zheng L, Chen B. Trimethylamine N-oxide in atherogenesis: impairing endothelial self-repair capacity and enhancing monocyte adhesion. Bioscience reports. 2017;37(2). doi:10.1042/BSR20160244.
Seldin MM, Meng Y, Qi H, Zhu W, Wang Z, Hazen SL, Lusis AJ, Shih DM. Trimethylamine N‐oxide promotes vascular inflammation through signaling of mitogen‐activated protein kinase and nuclear factor‐κB. Journal of the American Heart Association. 2016;5(2). doi:10.1161/JAHA.115.002767.
• Wang Z, Tang WH, Buffa JA, et al. Prognostic value of choline and betaine depends on intestinal microbiota-generated metabolite trimethylamine-N-oxide. Eur Heart J. 2014;35(14):904–10. Clinical study which highlights the importance of studying TMAO dietary precursors in the context of its microbiota conversion to TMAO.
•• Tang WH, Wang Z, Levison BS, et al. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med. 2013;368(17):1575–84. Seminal study which first linked TMAO with adverse cardiovascular outcomes in a large human cohort independent of traditional risk factors.
Meyer KA, Benton TZ, Bennett BJ, Jacobs DR, Lloyd‐Jones DM, Gross MD, Carr JJ, Gordon‐Larsen P, Zeisel SH. Microbiota‐Dependent Metabolite Trimethylamine N‐Oxide and Coronary Artery Calcium in the Coronary Artery Risk Development in Young Adults Study (CARDIA). Journal of the American Heart Association. 2016;5(10). doi:10.1161/JAHA.116.003970.
Li XS, Obeid S, Klingenberg R, et al. Gut microbiota-dependent trimethylamine N-oxide in acute coronary syndromes: a prognostic marker for incident cardiovascular events beyond traditional risk factors. Eur Heart J. 2017;38(11):814–24.
Senthong V, Li XS, Hudec T, et al. Plasma trimethylamine N-oxide, a gut microbe-generated phosphatidylcholine metabolite, is associated with atherosclerotic burden. J Am Coll Cardiol. 2016;67(22):2620–8.
Senthong V, Wang Z, Li XS, Fan Y, Wu Y, Tang WW, Hazen SL. Intestinal Microbiota‐Generated Metabolite Trimethylamine‐N‐Oxide and 5‐Year Mortality Risk in Stable Coronary Artery Disease: The Contributory Role of Intestinal Microbiota in a COURAGE‐Like Patient Cohort. Journal of the American Heart Association. 2016;5(6). doi:10.1161/JAHA.115.002816.
Mueller DM, Allenspach M, Othman A, et al. Plasma levels of trimethylamine-N-oxide are confounded by impaired kidney function and poor metabolic control. Atherosclerosis. 2015;243(2):638–44.
•• Zhu W, Gregory JC, Org E, et al. Gut microbial metabolite TMAO enhances platelet hyperreactivity and thrombosis risk. Cell. 2016;165(1):111–24. Key paper to uncover potential cardiovascular risk associated with TMAO-mediated platelet hyperresponsiveness.
Senthong V, Wang Z, Fan Y, Wu Y, Hazen SL, Tang WW. Trimethylamine N‐Oxide and Mortality Risk in Patients With Peripheral Artery Disease. Journal of the American Heart Association. 2016;5(10). doi:10.1161/JAHA.116.004237.
Mafune A, Iwamoto T, Tsutsumi Y, et al. Associations among serum trimethylamine-N-oxide (TMAO) levels, kidney function and infarcted coronary artery number in patients undergoing cardiovascular surgery: a cross-sectional study. Clin Exp Nephrol. 2016;20(5):731–9.
Skagen K, Troseid M, Ueland T, et al. The carnitine-butyrobetaine-trimethylamine-N-oxide pathway and its association with cardiovascular mortality in patients with carotid atherosclerosis. Atherosclerosis. 2016;247:64–9.
Tang WH, Wang Z, Kennedy DJ, et al. Gut microbiota-dependent trimethylamine N-oxide (TMAO) pathway contributes to both development of renal insufficiency and mortality risk in chronic kidney disease. Circ Res. 2015;116(3):448–55.
Stubbs JR, House JC, Ocque AJ, et al. Serum trimethylamine-N-oxide is elevated in CKD and correlates with coronary atherosclerosis burden. J Am Soc Nephrol. 2016;27(1):305–13.
Shafi T, Powe NR, Meyer TW, et al. Trimethylamine N-oxide and cardiovascular events in hemodialysis patients. J Am Soc Nephrol : JASN. 2017;28(1):321–31.
Eknoyan G, Beck GJ, Cheung AK, et al. Effect of dialysis dose and membrane flux in maintenance hemodialysis. N Engl J Med. 2002;347(25):2010–9.
Kim RB, Morse BL, Djurdjev O, et al. Advanced chronic kidney disease populations have elevated trimethylamine N-oxide levels associated with increased cardiovascular events. Kidney Int. 2016;89(5):1144–52.
Missailidis C, Hallqvist J, Qureshi AR, et al. Serum trimethylamine-N-oxide is strongly related to renal function and predicts outcome in chronic kidney disease. PLoS One. 2016;11(1):e0141738.
Kaysen GA, Johansen KL, Chertow GM, et al. Associations of trimethylamine N-oxide with nutritional and inflammatory biomarkers and cardiovascular outcomes in patients new to dialysis. J Ren Nutr. 2015;25(4):351–6.
Robinson-Cohen C, Newitt R, Shen DD, et al. Association of FMO3 variants and trimethylamine N-oxide concentration, disease progression, and mortality in CKD patients. PLoS One. 2016;11(8):e0161074.
Tang WH, Wang Z, Fan Y, et al. Prognostic value of elevated levels of intestinal microbe-generated metabolite trimethylamine-N-oxide in patients with heart failure: refining the gut hypothesis. J Am Coll Cardiol. 2014;64(18):1908–14.
Tang WH, Wang Z, Shrestha K, et al. Intestinal microbiota-dependent phosphatidylcholine metabolites, diastolic dysfunction, and adverse clinical outcomes in chronic systolic heart failure. J Card Fail. 2015;21(2):91–6.
Troseid M, Ueland T, Hov JR, et al. Microbiota-dependent metabolite trimethylamine-N-oxide is associated with disease severity and survival of patients with chronic heart failure. J Intern Med. 2015;277(6):717–26.
Suzuki T, Heaney LM, Bhandari SS, Jones DJ, Ng LL. Trimethylamine N-oxide and prognosis in acute heart failure. Heart. 2016;102(11):841–8.
Tang WH, Wang Z, Li XS, et al. Increased trimethylamine N-oxide portends high mortality risk independent of glycemic control in patients with type 2 diabetes mellitus. Clin Chem. 2017;63(1):297–306.
Lever M, George PM, Slow S, et al. Betaine and trimethylamine-N-oxide as predictors of cardiovascular outcomes show different patterns in diabetes mellitus: an observational study. PLoS One. 2014;9(12):e114969.
Mente A, Chalcraft K, Ak H, et al. The relationship between trimethylamine-N-oxide and prevalent cardiovascular disease in a multiethnic population living in Canada. Can J Cardiol. 2015;31(9):1189–94.
Randrianarisoa E, Lehn-Stefan A, Wang X, et al. Relationship of serum trimethylamine N-oxide (TMAO) levels with early atherosclerosis in humans. Sci Rep. 2016;6:26745.
Heianza Y, Ma W, Manson JE, Rexrode KM, Qi L. Gut Microbiota Metabolites and Risk of Major Adverse Cardiovascular Disease Events and Death: A Systematic Review and Meta‐Analysis of Prospective Studies. Journal of the American Heart Association. 2017;6(7). doi:10.1161/JAHA.116.004947
Zhu W, Wang Z, Tang WHW, Hazen SL. Gut microbe-generated trimethylamine N-oxide from dietary choline is prothrombotic in subjects. Circulation. 2017;135(17):1671–3.
Gregory JC, Buffa JA, Org E, et al. Transmission of atherosclerosis susceptibility with gut microbial transplantation. J Biol Chem. 2015;290(9):5647–60.
Liberles SD, Buck LB. A second class of chemosensory receptors in the olfactory epithelium. Nature. 2006;442(7103):645–50.
Ufnal M, Jazwiec R, Dadlez M, Drapala A, Sikora M, Skrzypecki J. Trimethylamine-N-oxide: a carnitine-derived metabolite that prolongs the hypertensive effect of angiotensin II in rats. Can J Cardiol. 2014;30(12):1700–5.
Yazdekhasti N, Brandsch C, Schmidt N, et al. Fish protein increases circulating levels of trimethylamine-N-oxide and accelerates aortic lesion formation in apoE null mice. Mol Nutr Food Res. 2016;60(2):358–68.
Vidal-Casariego A, Burgos-Pelaez R, Martinez-Faedo C, et al. Metabolic effects of L-carnitine on type 2 diabetes mellitus: systematic review and meta-analysis. Exp Clin Endocrinol Diabetes. 2013;121(4):234–8.
Mynatt RL. Carnitine and type 2 diabetes. Diabetes Metab Res Rev. 2009;25(Suppl 1):S45–9.
Collins HL, Drazul-Schrader D, Sulpizio AC, et al. L-Carnitine intake and high trimethylamine N-oxide plasma levels correlate with low aortic lesions in ApoE(−/−) transgenic mice expressing CETP. Atherosclerosis. 2016;244:29–37.
Bennett BJ, de Aguiar Vallim TQ, Wang Z, et al. Trimethylamine-N-oxide, a metabolite associated with atherosclerosis, exhibits complex genetic and dietary regulation. Cell Metab. 2013;17(1):49–60.
Thompson WG, Hensrud DD, Murad MH. Regarding L-carnitine and cardiovascular disease. Mayo Clin Proc. 2013;88(8):899–900.
Tang WH, Hazen SL. Reply: trimethylamine-N-oxide and heart failure. J Am Coll Cardiol. 2015;66(1):96–7.
Tang WH, Hazen SL. Reply: trimethylamine N-oxide in seafood: rotten or forgotten? J Am Coll Cardiol. 2016;68(25):2917–8.
Jovel J, Patterson J, Wang W, et al. Characterization of the gut microbiome using 16S or shotgun metagenomics. Front Microbiol. 2016;7:459.
Karlsson FH, Fak F, Nookaew I, et al. Symptomatic atherosclerosis is associated with an altered gut metagenome. Nat Commun. 2012;3:1245.
Lindskog Jonsson A, Hallenius FF, Akrami R, et al. Bacterial profile in human atherosclerotic plaques. Atherosclerosis. 2017;263:177–83.
Mitra S, Drautz-Moses DI, Alhede M, et al. In silico analyses of metagenomes from human atherosclerotic plaque samples. Microbiome. 2015;3:38.
• Falony G, Vieira-Silva S, Raes J. Microbiology meets big data: the case of gut microbiota-derived trimethylamine. Annu Rev Microbiol. 2015;69:305–21. Review which provides a framework for the integration of big data with functional microbiology.
Craciun S, Balskus EP. Microbial conversion of choline to trimethylamine requires a glycyl radical enzyme. Proc Natl Acad Sci U S A. 2012;109(52):21307–12.
Zhu Y, Jameson E, Crosatti M, et al. Carnitine metabolism to trimethylamine by an unusual Rieske-type oxygenase from human microbiota. Proc Natl Acad Sci U S A. 2014;111(11):4268–73.
Koeth RA, Levison BS, Culley MK, et al. gamma-Butyrobetaine is a proatherogenic intermediate in gut microbial metabolism of L-carnitine to TMAO. Cell Metab. 2014;20(5):799–812.
Meyer M, Granderath K, Andreesen JR. Purification and characterization of protein PB of betaine reductase and its relationship to the corresponding proteins glycine reductase and sarcosine reductase from Eubacterium acidaminophilum. Eur J Biochem. 1995;234(1):184–91.
Wagner M, Sonntag D, Grimm R, et al. Substrate-specific selenoprotein B of glycine reductase from Eubacterium acidaminophilum. Biochemical and molecular analysis. Eur J Biochem. 1999;260(1):38–49.
Mejean V, Iobbi-Nivol C, Lepelletier M, Giordano G, Chippaux M, Pascal MC. TMAO anaerobic respiration in Escherichia coli: involvement of the tor operon. Mol Microbiol. 1994;11(6):1169–79.
Martínez-del Campo A, Bodea S, Hamer HA, Marks JA, Haiser HJ, Turnbaugh PJ, Balskus EP. Characterization and detection of a widely distributed gene cluster that predicts anaerobic choline utilization by human gut bacteria. MBio. 2015;6(2). doi:10.1128/mBio.00042-15.
• Romano KA, Vivas EI, Amador-Noguez D, Rey FE. Intestinal microbiota composition modulates choline bioavailability from diet and accumulation of the proatherogenic metabolite trimethylamine-N-oxide. MBio. 2015;6(2):e02481. Study which demonstrates the utility of a gnotobiotic mouse model to systematically probe the functional relevance of bacterial species.
•• Rath S, Heidrich B, Pieper DH, Vital M. Uncovering the trimethylamine-producing bacteria of the human gut microbiota. Microbiome. 2017;5(1):54. Demonstration of a comprehensive analytic framework using metagenomics for the quantification and functional characterization of TMA-producing microbiota.
Brugere JF, Borrel G, Gaci N, Tottey W, O'Toole PW, Malpuech-Brugere C. Archaebiotics: proposed therapeutic use of archaea to prevent trimethylaminuria and cardiovascular disease. Gut Microbes. 2014;5(1):5–10.
Borrel G, McCann A, Deane J, Neto MC, Lynch DB, Brugère JF, O'Toole PW. Genomics and metagenomics of trimethylamine-utilizing Archaea in the human gut microbiome. The ISME Journal. 2017 6;11(9):2059-2074.
Chen ML, Yi L, Zhang Y, et al. Resveratrol attenuates trimethylamine-N-oxide (TMAO)-induced atherosclerosis by regulating TMAO synthesis and bile acid metabolism via remodeling of the gut microbiota. MBio. 2016;7(2):e02210–5.
Li J, Lin S, Vanhoutte PM, Woo CW, Xu A. Akkermansia muciniphila protects against atherosclerosis by preventing metabolic endotoxemia-induced inflammation in Apoe−/− mice. Circulation. 2016;133(24):2434–46.
Boutagy NE, Neilson AP, Osterberg KL, et al. Probiotic supplementation and trimethylamine-N-oxide production following a high-fat diet. Obesity (Silver Spring). 2015;23(12):2357–63.
• Wang Z, Roberts AB, Buffa JA, et al. Non-lethal inhibition of gut microbial trimethylamine production for the treatment of atherosclerosis. Cell. 2015;163(7):1585–95. Proof-of-concept demonstration of specific bacterial inhibition of TMAO production resulting in attenuated atherosclerosis.
Kuka J, Liepinsh E, Makrecka-Kuka M, et al. Suppression of intestinal microbiota-dependent production of pro-atherogenic trimethylamine N-oxide by shifting L-carnitine microbial degradation. Life Sci. 2014;117(2):84–92.
Funding
This research was supported by grants from the National Institutes of Health (NIH) and the Office of Dietary Supplements (P20HL113452, R01DK106000, R01HL126827). Mr. Li was supported by the Sarnoff Cardiovascular Research Fellowship.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Daniel Y. Li and W. H. Wilson Tang declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Genetics and Genomics
Rights and permissions
About this article
Cite this article
Li, D.Y., Tang, W.H.W. Gut Microbiota and Atherosclerosis. Curr Atheroscler Rep 19, 39 (2017). https://doi.org/10.1007/s11883-017-0675-9
Published:
DOI: https://doi.org/10.1007/s11883-017-0675-9