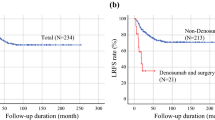
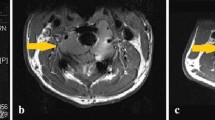
Opinion statement
Denosumab is a RANK ligand inhibitor approved for the treatment of giant cell tumor of bone. While the role of denosumab in the setting of advanced and unresectable disease is well established, its role in surgically resectable disease is currently under discussion. Several prospective and retrospective series on neoadjuvant therapy in potentially resectable tumor with high morbidity surgery reported a relapse rate of 10–20% after resection and 30–40% after curettage. At the same time, less morbid surgery has obvious clinical advantages for the patient, and several studies have shown the efficacy of denosumab in downgrading of the surgical procedure. Currently, the role of neoadjuvant denosumab in operable GCTB is limited to selected cases in which a diffuse reactive bone formation and peripheral ossification can make an easier surgical procedure, for example, in tumors with a large soft tissue component. A planned resection may become less morbid when preoperative denosumab is administered. Whenever a segmental resection is thought to be indicated at diagnosis, denosumab may be considered in the neoadjuvant setting. A preoperative course of 6 months is considered safe and effective. Two case scenarios are presented and critically discussed. Because of the high recurrence rates after denosumab treatment followed by curettage, we discourage the use of denosumab when curettage is considered feasible. In this setting, a short course of preoperative denosumab (2–6 months) may be considered for highly selected cases, for example in pathological fractures. The role of adjuvant denosumab needs further investigation. Long-term disease control has been reported in case of non-surgical lesions, even after treatment interruption, but there is no consensus on ideal treatment duration and dosage for these scenarios. In all cases, multidisciplinary discussion with oncology, pathologist, radiologist, and surgeons is mandatory. Patient’s comorbidities, dental conditions, and preferences, including family planning, should always be taken into account.


Similar content being viewed by others
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Thomas DM. RANKL, denosumab, and giant cell tumor of bone. Curr Opin Oncol. 2012;24(4):397–403.2.
Lewiecki EM. Clinical use of denosumab for the treatment for postmenopausal osteoporosis. Curr Med Res Opin. 2010;26(12):2807–12.
Branstetter DG, Nelson SD, Manivel JC, Blay JY, Chawla S, Thomas DM, et al. Denosumab induces tumor reduction and bone formation in patients with giant-cell tumor of bone. Clin Cancer Res. 2012;18(16):4415–24.
Thomas D, Carriere P, Jacobs I. Safety of denosumab in giant-cell tumour of bone. Lancet Oncol. 2010;11(9):815.
Martin-Broto J, Cleeland CS, Glare PA, Engellau J, Skubitz KM, Blum RH, et al. Effects of denosumab on pain and analgesic use in giant cell tumor of bone: interim results from a phase II study. Acta Oncol. 2014;53(9):1173–9.
Chawla S, Henshaw R, Seeger L, Choy E, Blay JY, Ferrari S, et al. Safety and efficacy of denosumab for adults and skeletally mature adolescents with giant cell tumour of bone: interim analysis of an open-label,parallel-group, phase 2 study. Lancet Oncol. 2013;14(9):901–8.
Traub F, Singh J, Dickson BC, Leung S, Mohankumar R, Blackstein ME, et al. Efficacy of denosumab in joint preservation for patients with giant cell tumour of the bone. Eur J Cancer. 2016;59:1–12.
Deveci MA, Paydaş S, Gönlüşen G, Özkan C, Biçer ÖS, Tekin M. Clinical and pathological results of denosumab treatment for giant cell tumors of bone: prospective study of 14 cases. Acta Orthop Traumatol Turc. 2017;51(1):1–6.
Aponte-Tinao L, Piuzzi NS, Roitman P, Farfalli GL. A high-grade sarcoma arising in a patient with recurrent benign giant cell tumor of the proximal tibia while receiving treatment with denosumab. Clin Orthop Relat Res. 2015;473:3050–5.
Broehm JC, Garbrecht EL, Wood J, Bocklage T. Two cases of sarcoma arising in giant cell tumor of bone treated with denosumab. Case Rep Med. 2015. https://doi.org/10.1155/2015/767198.
Alaqaili SI, Abduljabbar AM, Altaho AJ, Khan AA, Alherabi JA. Malignant sarcomatous transformation of benign giant cell tumor of bone after treatment with denosumab therapy: a literature review of reported cases. Cureus. 2018. https://doi.org/10.7759/cureus.3792.
Tsukamoto S, Righi A, Vanel D, Honoki K, Donati DM, Errani C. Development of high-grade osteosarcoma in a patient with recurrent giant cell tumor of the ischium while receiving treatment with denosumab. Jpn J Clin Oncol. 2017;47(11):1090–6.
Park A, Cipriano CA, Hill K, Kyriakos M, McDonald DJ. Malignant transformation of a giant cell tumor of bone treated with denosumab. JBJS Case Connect. 2016. https://doi.org/10.2106/JBJS.CC.16.00024.
Greenberg DD, Lee FY. Bisphosphonate-loaded bone cement as a local adjuvant therapy for giant cell tumor of bone a 1 to 12-year follow-up study. Am J Clin Oncol. 2019;42:231–7.
Balke M, Schremper L, Gebert C, Ahrens H, Streitbuerger A, Koehler G, et al. Giant cell tumor of bone: treatment and outcome of 214 cases. J Cancer Res Clin Oncol. 2008;134:969–78.
Knochentumoren A, Becker WT, Dohle J, et al. Local recurrence of giant cell tumor of bone after intralesional treatment with and without adjuvant therapy. J Bone Joint Surg Am. 2008;90:1060–7.
Algawahmed H, Turcotte R, Farrokhyar F, Ghert M. High-speed burring with and without the use of surgical adjuvants in the intralesional management of giant cell tumor of bone: a systematic review and meta-analysis. Sarcoma. 2010;2010:586090.
Kivioja AH, Blomqvist C, Hietaniemi K, Trovik C, Walloe A, Bauer HC, et al. Cement is recommended in intralesional surgery of giant cell tumors: a Scandinavian sarcoma group study of 294 patients followed for a median time of 5 years. Acta Orthop. 2008;79(1):86–93.
•• Chawla S, Blay JY, Rutkowski P, Le Cesne A, Reichardt P, Gelderblom H, et al. Denosumab in patients with giant-cell tumour of bone: a multicentre, open label, phase 2 study. Lancet Oncol. 2019;20(12):1719–29. Largest phase 2 clinical trial on denosumab for giant cell tumor of bone, including 532 patients, showing long-term efficacy and safety of the treatment.
Ueda T, Morioka H, Nishida Y, Kakunaga S, Tsuchiya H, Matsumoto Y, et al. Objective tumor response to denosumab in patients with giant cell tumor of bone: a multicenter phase II trial. Ann Oncol. 2015;26(10):2149–54.
Boye K, Jebsen NL, Zaikova O, Knobel H, Løndalen AM, Trovik CS, et al. Denosumab in patients with giant-cell tumor of bone in Norway: results from a nationwide cohort. Acta Oncol. 2017;56(3):479–83.
Rutkowski P, Ferrari S, Grimer RJ, Stalley PD, Dijkstra SP, Pienkowski A, et al. Surgical downstaging in an open-label phase II trial of denosumab in patients with giant cell tumor of bone. Ann Surg Oncol. 2015;22:2860–8.
Girolami I, Mancini I, Simoni A, Baldi GG, Simi L, Campanacci D, et al. Denosumab treated giant cell tumour of bone: a morphological, immunohistochemical and molecular analysis of a series. J Clin Pathol. 2016;69(3):240–7.
Errani C, Tsukamoto S, Leone G, Righi A, Akahane M, Tanaka Y, et al. Denosumab may increase the risk of local recurrence in patients with Giant-cell tumor of bone treated with curettage. J Bone Joint Surg Am. 2018;100(6):496–504.
• Luengo-Alonso G, Mellado-Romero M, Shemesh S, Ramos-Pascua L, Pretell-Mazzini J. Denosumab treatment for giant-cell tumor of bone: a systematic review of the literature. Arch Orthop Trauma Surg. 2019;139(10):1339–49. Systematic review of the literature including 1095 patients, showing clinical and radiologic responses, and less morbid surgery after denosumab.
Scoccianti G, Totti F, Scorianz M, Baldi G, Roselli G, Beltrami G, et al. Preoperative denosumab with curettage and cryotherapy in giant cell tumor of bone: is there an increased risk of local recurrence? Clin Orthop Relat Res. 2018;476(9):1783–90.
Agarwal MG, Gundavda MK, Gupta R, Reddy R. Does denosumab change the giant cell tumor treatment strategy? Lessons learned from early experience. Clin Orthop Relat Res. 2018;476(9):1773–82.
Puri A, Gulia A, Hegde P, Verma V, Rekhi B. Neoadjuvant denosumab: its role and results in operable cases of giant cell tumour of bone. Bone Joint J. 2019;101-B(2):170–7.
Müller DA, Beltrami G, Scoccianti G, Campanacci DA, Franchi A, Capanna R. Risks and benefits of combining denosumab and surgery in giant cell tumor of bone-a case series. World J Surg Oncol. 2016;14:281.
CDM F, Unni KK, Mertens F, World Health Organization, International Agency for Research on Cancer. Pathology and genetics of tumours of soft tissue and bone. Geneva: IARC Press; 2002. p. 427.
Mendenhall WM, Zlotecki RA, Scarborough MT, Gibbs CP, Mendenhall NP. Giant cell tumor of bone. Am J Clin Oncol. 2006;29(1):96–9 Review.
Chinder PS, Hindiskere S, Doddarangappa S, Pal U. Evaluation of local recurrence in giant-cell tumor of bone treated by neoadjuvant denosumab. Clin Orthop Surg. 2019;11:352–60.
• Rutkowski P, Gaston L, Borkowska A, Stacchiotti S, Gelderblom H, Baldi GG, et al. Denosumab treatment of inoperable or locally advanced giant cell tumor of bone - Multicenter analysis outside clinical trial. Eur J Surg Oncol. 2018;44:1384–90. Multicenter study including 138 patients showing the surgical downstaging effect of denosumab.
Author information
Authors and Affiliations
Contributions
EP had the idea for the article, ES and LBJ performed the literature search and data analysis, and all drafted and/or critically revised the work.
Corresponding author
Ethics declarations
Conflict of Interest
Emanuela Palmerini has received compensation from Daiichi Sankyo, Deciphera, Lilly, and EUSA Pharma for service as a consultant and has received non-financial support from PharmaMar.
Eric Lodewijk Staals declares that he has no conflict of interest.
Louis Baxter Jones declares that he has no conflict of interest.
Davide Maria Donati declares that he has no conflict of interest.
Alessandra Longhi declares that she has no conflict of interest.
R. Lor Randall declares that he has no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Sarcoma
Rights and permissions
About this article
Cite this article
Palmerini, E., Staals, E.L., Jones, L.B. et al. Role of (Neo)adjuvant Denosumab for Giant Cell Tumor of Bone. Curr. Treat. Options in Oncol. 21, 68 (2020). https://doi.org/10.1007/s11864-020-00766-4
Published:
DOI: https://doi.org/10.1007/s11864-020-00766-4