Opinion Statement
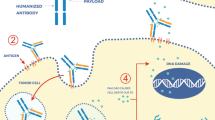
Antibody-drug conjugates are an elegant approach to cancer treatment that couples the specificity of monoclonal antibodies to the cytotoxicity of classic chemotherapy agents, permitting, at least in theory, increased activity and reduced toxicity. In breast cancer, the early success of trastuzumab-emtansine (T-DM1) in the HER2-positive metastatic setting led to great hopes, later dashed by results in the early setting (KRISTINE trial) and in combination with pertuzumab (MARIANNE trial). Parallel to this, development of ADCs in breast cancer has suffered other setbacks, including the recent failure of other agents (MM-302) as well as the suspension of a few programs (XMT-1522, ADCT-502) with the overall effect of dampening the impetus of this concept and halting/delaying the progress of drugs associated with it, particularly when immunotherapy is at the center of so many efforts. Numerous antibody-drug conjugates remain, however, in development, and could prove successful. Critically, ADCs could permit the introduction of novel concepts such as the expansion of potent anti-HER2 therapy to HER2-low breast cancer, treatment beyond resistance to T-DM1, and synergy in combination with immune checkpoint blockade. In the early setting, the ATEMPT trial may show that T-DM1 reduces toxicity while maintaining good outcomes for lower risk HER2+ patients. ADCs based on bispecific antibodies are also in development. Finally, breakthroughs are occurring in the orphan triple-negative breast cancer subtype with agents targeting surface proteins. The recent results of Sacituzumab govitecan suggest substantial activity in heavily pre-treated patients and underscore the enduring relevance of antibody drug conjugates as a path towards better outcomes.

Similar content being viewed by others
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–86.
Cancer of the Breast (Female) - SEER Stat Fact Sheets [Internet]. [cited 2016 Oct 24]. Available from: http://seer.cancer.gov/statfacts/html/breast.html. Accessed 15 Sept 2018.
Cardoso F, Costa A, Senkus E, Aapro M, André F, Barrios CH, et al. 3rd ESO–ESMO International Consensus Guidelines for Advanced Breast Cancer (ABC 3). Ann Oncol. 2016;mdw544.
Sonnenblick A, Pondé N, Piccart M. Metastatic breast cancer: the Odyssey of personalization. Mol Oncol. 2016;10(8):1147–59.
Pegram M, Hsu S, Lewis G, Pietras R, Beryt M, Sliwkowski M, et al. Inhibitory effects of combinations of HER-2/neu antibody and chemotherapeutic agents used for treatment of human breast cancers. Oncogene. 1999;18(13):2241–51.
Giansanti P, Preisinger C, Huber KVM, Gridling M, Superti-Furga G, Bennett KL, et al. Evaluating the promiscuous nature of tyrosine kinase inhibitors assessed in A431 epidermoid carcinoma cells by both chemical- and phosphoproteomics. ACS Chem Biol. 2014;9(7):1490–8.
Tolcher AW. Antibody drug conjugates: lessons from 20 years of clinical experience. Ann Oncol. 2016;27(12):2168–72.
Moek KL, de Groot DJA, de Vries EGE, Fehrmann RSN. The antibody–drug conjugate target landscape across a broad range of tumour types. Ann Oncol. 2017;28(12):3083–91.
Beck A, Goetsch L, Dumontet C, Corvaïa N. Strategies and challenges for the next generation of antibody–drug conjugates. Nat Rev Drug Discov. 2017;16(5):315–37.
Damelin M, Zhong W, Myers J, Sapra P. Evolving strategies for target selection for antibody-drug conjugates. Pharm Res. 2015;32(11):3494–507.
Visintin A, Knowlton K, Tyminski E, Lin C-I, Zheng X, Marquette K, et al. Novel anti-TM4SF1 antibody-drug conjugates with activity against tumor cells and tumor vasculature. Mol Cancer Ther. 2015;14(8):1868–76.
Gerlinger M, Rowan AJ, Horswell S, Larkin J, Endesfelder D, Gronroos E, et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med. 2012;366(10):883–92.
Issell BF, Crooke ST. Maytansine. Cancer Treat Rev. 1978;5(4):199–207.
Cianfriglia M. The biology of MDR1-P-glycoprotein (MDR1-Pgp) in designing functional antibody drug conjugates (ADCs): the experience of gemtuzumab ozogamicin. Ann Ist Super Sanita. 2013;49(2):150–68.
McCombs JR, Owen SC. Antibody drug conjugates: design and selection of linker, Payload and Conjugation Chemistry. AAPS J. 2015;17(2):339–51.
Staudacher AH, Brown MP. Antibody drug conjugates and bystander killing: is antigen-dependent internalisation required? Br J Cancer. 2017;117(12):1736–42.
Masters JC, Nickens DJ, Xuan D, Shazer RL, Amantea M. Clinical toxicity of antibody drug conjugates: a meta-analysis of payloads. Investig New Drugs. 2018;36(1):121–35.
Ait-Oudhia S, Zhang W, Mager DE. A mechanism-based PK/PD model for hematological toxicities induced by antibody-drug conjugates. AAPS J. 2017;19(5):1436–48.
Ravry MJ, Omura GA, Birch R. Phase II evaluation of maytansine (NSC 153858) in advanced cancer. A Southeastern Cancer Study Group trial. Am J Clin Oncol. 1985;8(2):148–50.
Lambert JM, Chari RVJ. Ado-trastuzumab emtansine (T-DM1): an antibody–drug conjugate (ADC) for HER2-positive breast cancer. J Med Chem. 2014;57(16):6949–64.
Verma S, Miles D, Gianni L, Krop IE, Welslau M, Baselga J, et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med. 2012;367(19):1783–91.
•• Diéras V, Miles D, Verma S, Pegram M, Welslau M, Baselga J, et al. Trastuzumab emtansine versus capecitabine plus lapatinib in patients with previously treated HER2-positive advanced breast cancer (EMILIA): a descriptive analysis of final overall survival results from a randomised, open-label, phase 3 trial. Lancet Oncol. 2017;18(6):732–42 Updated results of the pivotal EMILIA trial.
Welslau M, Diéras V, Sohn J-H, Hurvitz SA, Lalla D, Fang L, et al. Patient-reported outcomes from EMILIA, a randomized phase 3 study of trastuzumab emtansine (T-DM1) versus capecitabine and lapatinib in human epidermal growth factor receptor 2-positive locally advanced or metastatic breast cancer: PROs from phase 3 T-DM1 HER2+ MBC study. Cancer. 2014;120(5):642–51.
Krop IE, Kim S-B, González-Martín A, LoRusso PM, Ferrero J-M, Smitt M, et al. Trastuzumab emtansine versus treatment of physician’s choice for pretreated HER2-positive advanced breast cancer (TH3RESA): a randomised, open-label, phase 3 trial. Lancet Oncol. 2014;15(7):689–99.
•• Krop IE, Kim S-B, Martin AG, LoRusso PM, Ferrero J-M, Badovinac-Crnjevic T, et al. Trastuzumab emtansine versus treatment of physician’s choice in patients with previously treated HER2-positive metastatic breast cancer (TH3RESA): final overall survival results from a randomised open-label phase 3 trial. Lancet Oncol. 2017;18(6):743–54 Updated results of the pivotal TH3RESA trial.
Bartley K, Wildiers H, Kim S-B, Krop IE, Kang J, Yu R, et al. Patient-reported outcomes (PROs) from TH3RESA, a phase 3 study of trastuzumab emtansine (T-DM1) versus treatment of physician’s choice (TPC) in patients with pretreated HER2-positive advanced breast cancer. J Clin Oncol. 2014;32(26_suppl):153.
Perez EA, Barrios C, Eiermann W, Toi M, Im Y-H, Conte P, et al. Trastuzumab emtansine with or without pertuzumab versus trastuzumab plus taxane for human epidermal growth factor receptor 2-positive, advanced breast cancer: primary results from the phase III MARIANNE study. J Clin Oncol. 2017;35(2):141–8.
von Minckwitz G, Huang C-S, Mano MS, Loibl S, Mamounas EP, Untch M, et al. Trastuzumab emtansine for residual invasive HER2-positive breast cancer. N Engl J Med [Internet]. 2018 5 [cited 2018 Dec 10]; Available from: http://www.nejm.org/doi/10.1056/NEJMoa1814017. Accessed 15 Sept 2018.
Hurvitz SA, Martin M, Symmans WF, Jung KH, Huang C-S, Thompson AM, et al. Neoadjuvant trastuzumab, pertuzumab, and chemotherapy versus trastuzumab emtansine plus pertuzumab in patients with HER2-positive breast cancer (KRISTINE): a randomised, open-label, multicentre, phase 3 trial. Lancet Oncol [Internet]. 2017 Nov [cited 2017 Nov 30]; Available from: http://linkinghub.elsevier.com/retrieve/pii/S1470204517307167. Accessed 15 Sept 2018.
Burris HA, Rugo HS, Vukelja SJ, Vogel CL, Borson RA, Limentani S, et al. Phase II study of the antibody drug conjugate trastuzumab-DM1 for the treatment of human epidermal growth factor receptor 2 (HER2)-positive breast cancer after prior HER2-directed therapy. J Clin Oncol Off J Am Soc Clin Oncol. 2011;29(4):398–405.
Krop IE, LoRusso P, Miller KD, Modi S, Yardley D, Rodriguez G, et al. A phase II study of trastuzumab emtansine in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer who were previously treated with trastuzumab, lapatinib, an anthracycline, a taxane, and capecitabine. J Clin Oncol Off J Am Soc Clin Oncol. 2012;30(26):3234–41.
Krop IE, Beeram M, Modi S, Jones SF, Holden SN, Yu W, et al. Phase I study of trastuzumab-DM1, an HER2 antibody-drug conjugate, given every 3 weeks to patients with HER2-positive metastatic breast cancer. J Clin Oncol Off J Am Soc Clin Oncol. 2010;28(16):2698–704.
Hurvitz SA, Dirix L, Kocsis J, Bianchi GV, Lu J, Vinholes J, et al. Phase II randomized study of trastuzumab emtansine versus trastuzumab plus docetaxel in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer. J Clin Oncol Off J Am Soc Clin Oncol. 2013;31(9):1157–63.
• Pondé N, Brandão M, El-Hachem G, Werbrouck E, Piccart M. Treatment of advanced HER2-positive breast cancer: 2018 and beyond. Cancer Treat Rev. 2018;67:10–20 Complete overview of the current results of the field.
Swain SM, Baselga J, Kim S-B, Ro J, Semiglazov V, Campone M, et al. Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N Engl J Med. 2015;372(8):724–34.
Fabi A, Giannarelli D, Moscetti L, Santini D, Zambelli A, Laurentiis MD, et al. Ado-trastuzumab emtansine (T-DM1) in HER2+ advanced breast cancer patients: does pretreatment with pertuzumab matter? Future Oncol. 2017;13(30):2791–7.
Vici P, Pizzuti L, Michelotti A, Sperduti I, Natoli C, Mentuccia L, et al. A retrospective multicentric observational study of trastuzumab emtansine in HER2 positive metastatic breast cancer: a real-world experience. Oncotarget. 2017 22 [cited 2018 Jul 18];8(34). Available from: http://www.oncotarget.com/fulltext/18176. Accessed 15 Sept 2018.
Conte B. Effectiveness of trastuzumab emtansine (TDM1) in patients with HER2-positive advanced breast cancer (ABC) progressing after taxane plus pertuzumab plus trastuzumab. Ann Oncol. 2018;29(suppl_8):viii90–viii121. https://doi.org/10.1093/annonc/mdy272 ESMO 2018; Munich.
Schneeweiss A. Ado-trastuzumab for the treatment of metastatic HER2-amplified breast cancer patients previously treated with pertuzumab. SABCS 2018; San Antonio, Texas.
Kim S-B, Wildiers H, Krop IE, Smitt M, Yu R, Lysbet de Haas S, et al. Relationship between tumor biomarkers and efficacy in TH3RESA, a phase III study of trastuzumab emtansine (T-DM1) vs. treatment of physician’s choice in previously treated HER2-positive advanced breast cancer: Biomarker analyses in TH3RESA. Int J Cancer. 2016;139(10):2336–42.
Badve SS, Gokmen-Polar Y, Hoersch S, Xu J, Ruschoff J, de Haas S, et al. Role of tumor infiltrating lymphocytes (TILs) in HER2+ metastatic breast cancers (MBC) treated with trastuzumab emtansine (T-DM1) or lapatinib plus capecitabine (L+C) (EMILIA Trial). J Clin Oncol. 2016;34(15_suppl):607.
Baselga J, Lewis Phillips GD, Verma S, Ro J, Huober J, Guardino E, et al. Relationship between tumor biomarkers and efficacy in EMILIA, a phase III study of trastuzumab emtansine in HER2-positive metastatic breast cancer. Clin Cancer Res 2016 26.
Montemurro F, Ellis P, Delaloge S, Wuerstlein R, Anton A, Button P, et al. Safety and efficacy of trastuzumab emtansine (T-DM1) in 399 patients with central nervous system metastases: exploratory subgroup analysis from the KAMILLA study. San Antonio: SABCS; 2016.
Krop IE, Lin NU, Blackwell K, Guardino E, Huober J, Lu M, et al. Trastuzumab emtansine (T-DM1) versus lapatinib plus capecitabine in patients with HER2-positive metastatic breast cancer and central nervous system metastases: a retrospective, exploratory analysis in EMILIA. Ann Oncol. 2015;26(1):113–9.
Bartsch R, Berghoff AS, Vogl U, Rudas M, Bergen E, Dubsky P, et al. Activity of T-DM1 in Her2-positive breast cancer brain metastases. Clin Exp Metastasis. 2015;32(7):729–37.
Earl HM. PERSEPHONE: 6 versus 12 months (m) of adjuvant trastuzumab in patients (pts) with HER2 positive (+) early breast cancer (EBC): randomised phase 3 non-inferiority trial with definitive 4-year (yr) disease-free survival (DFS) results. Slide set. Chicago: ASCO; 2018.
Martin M, Holmes FA, Ejlertsen B, Delaloge S, Moy B, Iwata H, et al. Neratinib after trastuzumab-based adjuvant therapy in HER2-positive breast cancer (ExteNET): 5-year analysis of a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2017;18(12):1688–700.
von Minckwitz G, Procter M, de Azambuja E, Zardavas D, Benyunes M, Viale G, et al. Adjuvant pertuzumab and trastuzumab in early HER2-positive breast cancer. N Engl J Med. 2017;377(2):122–31.
Garrido-Laguna I. A phase I study of PF-06647263, a novel EFNA4-ADC, in patients with metastatic triple negative breast cancer. Chicago: ASCO; 2017.
Xu B. An open-label, multicenter, phase Ib study to evaluate RC48-ADC in patients with HER2-positive metastatic breast cancer. Chicago: ASCO; 2018.
Wang J. An open-label, dose-escalation phase I study to evaluate RC48-ADC, a novel antibody-drug conjugate, in patients with HER2-positive metastatic breast cancer. Chicago: ASCO; 2018.
Sachdev JC. PF-06647020 (PF-7020), an antibody-drug conjugate (ADC) targeting protein tyrosine kinase 7 (PTK7), in patients (pts) with advanced solid tumors:r of a phase I dose escalation and expansion study. Chicago: ASCO; 2018.
Modi S. Phase 1 study of the antibody-drug conjugate SGN-LIV1A in patients with heavily pretreated triple-negative metastatic breast cancer. Chicago: ASCO; 2018.
Gomez-Roca C. A phase I study of SAR566658, an anti CA6-antibody drug conjugate (ADC), in patients (Pts) with CA6-positive advanced solid tumors (STs) (NCT01156870). Chicago: ASCO; 2018.
Bardia A. Efficacy of sacituzumab govitecan (anti-Trop-2-SN-38 antibody-drug conjugate) for treatment-refractory hormone-receptor positive (HR+)/HER2− metastatic breast cancer (mBC). Chicago: ASCO; 2018.
Bardia A, Mayer IA, Diamond JR, Moroose RL, Isakoff SJ, Starodub AN, et al. Efficacy and safety of anti-Trop-2 antibody drug conjugate sacituzumab govitecan (IMMU-132) in heavily pretreated patients with metastatic triple-negative breast cancer. J Clin Oncol Off J Am Soc Clin Oncol. 2017;35(19):2141–8.
Bardia A. Sacituzumab govitecan (IMMU-132), an anti-Trop-2-SN-38 antibody-drug conjugate, as ≥3rd-line therapeutic option for patients with relapsed/refractory metastatic triple-negative breast cancer (mTNBC): efficacy results. San Antonio: SABCS; 2017.
Burris HA. A phase I/II study of CR011 vcMMAE (CDX 011), an antibody drug conjugate, in patients with locally advanced or metastatic breast cancer. San Antonio, Texas.
Pegram M. 47O - Phase 1 study of bispecific HER2 antibody-drug conjugate MEDI4276 in patients with advanced HER2-positive breast or gastric cancer. Paris: TAT; 2018.
Iwata H. Trastuzumab deruxtecan (DS-8201a) in subjects with HER2-expressing solid tumors: long-term results of a large phase 1 study with multiple expansion cohorts. Chicago: ASCO; 2018.
Saura C. A phase I expansion cohorts study of SYD985 in heavily pretreated patients with HER2-positive or HER2-low metastatic breast cancer. In Chicago, IL, USA.
Hamilton EP. Phase 1 dose escalation of XMT-1522, a novel HER2-targeting antibody-drug conjugate (ADC), in patients (pts) with HER2-expressing breast, lung and gastric tumors. Chicago: ASCO; 2018.
Fehrenbacher L. NSABP B-47 (NRG oncology): phase III randomized trial comparing adjuvant chemotherapy with adriamycin (A) and cyclophosphamide (C) → weekly paclitaxel (WP), or docetaxel (T) and C with or without a year of trastuzumab (H) in women with node-positive or high-risk node-negative invasive breast cancer (IBC) expressing HER2 staining intensity of IHC 1+ or 2+ with negative FISH (HER2-low IBC). In San Antonio, Texas;
Saura C. Primary results of LORELEI: a phase II randomised double-blind study of neoadjuvant letrozole plus taselisib versus letrozole plus placebo in postmenopausal women with ER-positive/HER2-negative early stage breast cancer. Madrid: ESMO; 2017.
Bardia A, Mayer IA, Vahdat LT, Tolaney SM, Isakoff SJ, Diamond JR, et al. Sacituzumab govitecan-hziy in refractory metastatic triple-negative breast cancer. N Engl J Med. 2019;380(8):741–51.
Bergstrom D. A novel, highly potent HER2-targeted antibody-drug conjugate (ADC) for the treatment of low HER2-expressing tumors and combination with trastuzumab-based regimens in HER2-driven tumors. Philadelphia: AACR; 2015.
Bergstrom D. XMT-1522 induces tumor regressions in preclinical models representing HER2 positive and HER2 low expressing breast cancer. San Antonio: SABCS; 2015.
van der Lee MMC, Groothuis PG, Ubink R, van der Vleuten MAJ, van Achterberg TA, Loosveld EM, et al. The preclinical profile of the duocarmycin-based HER2-targeting ADC SYD985 predicts for clinical benefit in low HER2-expressing breast cancers. Mol Cancer Ther. 2015;14(3):692–703.
Aftimos P. SYD985, a novel anti-HER2 ADC, shows promising activity in patients with HER2-positive and HER2-low metastatic breast cancer. San Antonio: SABCS; 2017.
Doi T, Iwata H, Tsurutani J, Takahashi S, Park H, Redfern CH, et al. Single agent activity of DS-8201a, a HER2-targeting antibody-drug conjugate, in heavily pretreated HER2 expressing solid tumors. J Clin Oncol. 2017;35(15_suppl):108.
Modi S. Safety and efficacy results from a phase 1 study of DS-8201a in patients with HER2 expressing breast cancers. San Antonio: SABCS; 2017.
Abstract P6-17-06: Characterization, monitoring and management of interstitial lung disease in patients with metastatic breast cancer: analysis of data available from multiple studies of DS-8201a, a HER2-targeted antibody drug conjugate with a topoisomer… | Cancer Res [Internet]. [cited 2019 Feb 26]. Available from: http://cancerres.aacrjournals.org/content/79/4_Supplement/P6-17-06. Accessed 15 Sept 2018.
Reynolds JG, Geretti E, Hendriks BS, Lee H, Leonard SC, Klinz SG, et al. HER2-targeted liposomal doxorubicin displays enhanced anti-tumorigenic effects without associated cardiotoxicity. Toxicol Appl Pharmacol. 2012;262(1):1–10.
LoRusso P, Krop I, Miller K, Ma C, Siegel BA, Shields AF, et al. Abstract CT234: a phase I study of MM-302, a HER2-targeted PEGylated liposomal doxorubicin, in patients with HER2+ metastatic breast cancer. Cancer Res. 2015;75(15 Supplement):CT234.
Miller K, Cortes J, Hurvitz SA, Krop IE, Tripathy D, Verma S, et al. HERMIONE: a randomized phase 2 trial of MM-302 plus trastuzumab versus chemotherapy of physician’s choice plus trastuzumab in patients with previously treated, anthracycline-naïve, HER2-positive, locally advanced/metastatic breast cancer. BMC Cancer [Internet]. 2016 Dec [cited 2017 Dec 5];16(1). Available from: http://bmccancer.biomedcentral.com/articles/10.1186/s12885-016-2385-z. Accessed 15 Sept 2018.
Li JY, Perry SR, Muniz-Medina V, Wang X, Wetzel LK, Rebelatto MC, et al. A biparatopic HER2-targeting antibody-drug conjugate induces tumor regression in primary models refractory to or ineligible for HER2-targeted therapy. Cancer Cell. 2016;29(1):117–29.
Yao X, Jiang J, Wang X, Huang C, Li D, Xie K, et al. A novel humanized anti-HER2 antibody conjugated with MMAE exerts potent anti-tumor activity. Breast Cancer Res Treat. 2015;153(1):123–33.
Goldenberg DM, Stein R, Sharkey RM. The emergence of trophoblast cell-surface antigen 2 (TROP-2) as a novel cancer target. Oncotarget [Internet]. 2018 Jun 22 [cited 2018 Jul 19];9(48). Available from: http://www.oncotarget.com/fulltext/25615. Accessed 15 Sept 2018.
Ocean AJ, Starodub AN, Bardia A, Vahdat LT, Isakoff SJ, Guarino M, et al. Sacituzumab govitecan (IMMU-132), an anti-Trop-2-SN-38 antibody-drug conjugate for the treatment of diverse epithelial cancers: Safety and pharmacokinetics. Cancer. 2017;123(19):3843–54.
Rose AAN, Grosset AA, Dong Z, Russo C, MacDonald PA, Bertos NR, et al. Glycoprotein nonmetastatic B is an independent prognostic indicator of recurrence and a novel therapeutic target in breast cancer. Clin Cancer Res. 2010;16(7):2147–56.
Maric G, Annis MG, Dong Z, Rose AAN, Ng S, Perkins D, et al. GPNMB cooperates with neuropilin-1 to promote mammary tumor growth and engages integrin α5β1 for efficient breast cancer metastasis. Oncogene. 2015;34:5494–504.
Rose AAN, Biondini M, Curiel R, Siegel PM. Targeting GPNMB with glembatumumab vedotin: current developments and future opportunities for the treatment of cancer. Pharmacol Ther. 2017;179:127–41.
Bendell J, Saleh M, Rose AAN, Siegel PM, Hart L, Sirpal S, et al. Phase I/II study of the antibody-drug conjugate glembatumumab vedotin in patients with locally advanced or metastatic breast cancer. J Clin Oncol Off J Am Soc Clin Oncol. 2014;32(32):3619–25.
Yardley DA, Weaver R, Melisko ME, Saleh MN, Arena FP, Forero A, et al. EMERGE: a randomized phase II study of the antibody-drug conjugate glembatumumab vedotin in advanced glycoprotein NMB-expressing breast cancer. J Clin Oncol Off J Am Soc Clin Oncol. 2015;33(14):1609–19.
Vahdat LT, Forero-Torres A, Schmid P, Blackwell K, Telli ML, Melisko M, et al. Abstract P6-20-01: METRIC: a randomized international phase 2b study of the antibody-drug conjugate (ADC) glembatumumab vedotin (GV) in gpNMB-overexpressing, metastatic, triple-negative breast cancer (mTNBC). Cancer Res. 2019;79(4 Supplement):P6-20-01.
Taylor KM, Morgan HE, Smart K, Zahari NM, Pumford S, Ellis IO, et al. The emerging role of the LIV-1 subfamily of zinc transporters in breast cancer. Mol Med. 2007;13(7–8):396–406.
Sussman D, Smith LM, Anderson ME, Duniho S, Hunter JH, Kostner H, et al. SGN-LIV1A: a novel antibody-drug conjugate targeting LIV-1 for the treatment of metastatic breast cancer. Mol Cancer Ther. 2014;13(12):2991–3000.
Damelin M, Bankovich A, Bernstein J, Lucas J, Chen L, Williams S, et al. A PTK7-targeted antibody-drug conjugate reduces tumor-initiating cells and induces sustained tumor regressions. Sci Transl Med. 2017;9(372):eaag2611.
Lisabeth EM, Falivelli G, Pasquale EB. Eph receptor signaling and ephrins. Cold Spring Harb Perspect Biol. 2013;5(9):–a009159.
Barquilla A, Pasquale EB. Eph receptors and ephrins: therapeutic opportunities. Annu Rev Pharmacol Toxicol. 2015;55(1):465–87.
Damelin M, Bankovich A, Park A, Aguilar J, Anderson W, Santaguida M, et al. Anti-EFNA4 calicheamicin conjugates effectively target triple-negative breast and ovarian tumor-initiating cells to result in sustained tumor regressions. Clin Cancer Res. 2015;21(18):4165–73.
Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science. 2018;359(6382):1350–5.
Loi S, Giobbie-Hurder A, Gombos A, Bachelot T, Hui R, Curigliano G, et al. Pembrolizumab plus trastuzumab in trastuzumab-resistant, advanced, HER2-positive breast cancer (PANACEA): a single-arm, multicentre, phase 1b–2 trial. Lancet Oncol [Internet]. 2019 Feb [cited 2019 Feb 26]; Available from: https://linkinghub.elsevier.com/retrieve/pii/S147020451830812X. Accessed 15 Sept 2018.
Schmid P, Adams S, Rugo HS, Schneeweiss A, Barrios CH, Iwata H, et al. Atezolizumab and Nab-paclitaxel in advanced triple-negative breast cancer. N Engl J Med. 2018;379(22):2108–21.
Swoboda A, Nanda R. Immune checkpoint blockade for breast cancer. Cancer Treat Res. 2018;173:155–65.
Martin K, Schreiner J, Zippelius A. Modulation of APC function and anti-tumor immunity by anti-cancer drugs. Front Immunol [Internet]. 2015 Sep 29 [cited 2018 Aug 1];6. Available from: http://journal.frontiersin.org/Article/10.3389/fimmu.2015.00501/abstract. Accessed 15 Sept 2018.
Müller P, Kreuzaler M, Khan T, Thommen DS, Martin K, Glatz K, et al. Trastuzumab emtansine (T-DM1) renders HER2+ breast cancer highly susceptible to CTLA-4/PD-1 blockade. Sci Transl Med. 2015;7(315):315ra188.
Emens LA, Esteva F, Beresford M, Saura C, Laurentiis MD, Kim S-B, et al. Abstract PD3-01: Results from KATE2, a randomized phase 2 study of atezolizumab (atezo)+trastuzumab emtansine (T-DM1) vs placebo (pbo)+T-DM1 in previously treated HER2+ advanced breast cancer (BC). Cancer Res. 2019;79(4 Supplement):PD3-01.
Gebhart G, Flamen P, De Vries EGE, Jhaveri K, Wimana Z. Imaging diagnostic and therapeutic targets: human epidermal growth factor receptor 2. J Nucl Med. 2016;57(Suppl 1):81S–8S.
Gebhart G, Lamberts LE, Wimana Z, Garcia C, Emonts P, Ameye L, et al. Molecular imaging as a tool to investigate heterogeneity of advanced HER2-positive breast cancer and to predict patient outcome under trastuzumab emtansine (T-DM1): the ZEPHIR trial. Ann Oncol. 2016;27(4):619–24.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Noam Pondé has received travel support from Roche/Genentech, Janssen-Cilag, and Mundipharma, as well as speaker’s fees from Mundipharma. The institute he works for has received research funding from AstraZeneca, Lilly, MSD, Novartis, Pfizer, Roche-Genentech, Synthon, Radius, and Servier. Philippe Aftimos has received travel support from Roche, MSD, and Amgen; consulting fees from Synthon, Boehringer/Ingleheim, and Macrogenics; and speaker Fees from Amgen and Novartis. The institute he works for has received research funding from AstraZeneca, Lilly, MSD, Novartis, Pfizer, Roche-Genentech, Synthon, Radius, and Servier. Martine Piccart is a board member of Radius. She has received honoraria as a consultant from AstraZeneca, Lilly, MSD, Novartis, Odonate, Pfizer, Roche-Genentech, Camel-IDS, Crescendo Biologics, Periphagen, Huya, Debiopharm, PharmaMar, G1 Therapeutics, Menarini, Seattle Genetics, Immunomedics, and Oncolytics. Her institute has received research funding from AstraZeneca, Lilly, MSD, Novartis, Pfizer, Roche-Genentech, Synthon, Radius, and Servier.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Breast Cancer
Rights and permissions
About this article
Cite this article
Pondé, N., Aftimos, P. & Piccart, M. Antibody-Drug Conjugates in Breast Cancer: a Comprehensive Review. Curr. Treat. Options in Oncol. 20, 37 (2019). https://doi.org/10.1007/s11864-019-0633-6
Published:
DOI: https://doi.org/10.1007/s11864-019-0633-6