Abstract
Background
The pathogenesis of Hirschprung’s disease (HSCR) remains largely unknown. The lncRNA ZNFX1 antisense RNA 1 (ZFAS1) has been found to have vital regulatory roles in a number of diseases. However, the association between ZFAS1 and HSCR has not been reported.
Aims
The present study was aimed at investigating the expression pattern and biological function and underlying mechanisms of ZFAS1 in HSCR.
Methods
The expression of ZFAS1 was detected in surgical excision samples of 30 children diagnosed with HSCR and 30 control cases. Functional experiments were conducted after over-expression or knockdown of ZFAS1 in human neuronal cell line SH-SY-5Y. Multiple bioinformatics databases and tools were used to explore the potential regulatory mechanisms of ZFAS1 in HSCR.
Results
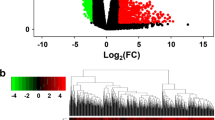
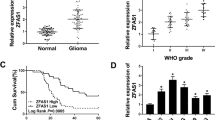
Compared with the control group, the HSCR group has a significantly higher level of ZFAS1(P = 0.0012). The area under the curve (AUC) of the receiver operating characteristic (ROC) curve was 0.7133 (P = 0.0045), which indicated good biomarker potency of ZFAS1 in HSCR. Functionally, over-expression of ZFAS1 significantly inhibited cell proliferation, whereas knockdown of ZFAS1 promoted cell proliferation and colony formation of SH-SY-5Y cells. Using multiple databases, a competing endogenous RNA (ceRNA) network, containing ZFAS1,13 candidate miRNAs, and 110 potential gene targets, was established. Further enrichment analysis suggested that ZFAS1 may regulate a number of genes and signaling pathways that were crucial for neuron development.
Conclusions
Our findings revealed that ZFAS1 may participate in the pathogenesis of HSCR through regulating neuron functions. Bioinformatics analysis highlighted an important perspective for the following mechanical researches.







Similar content being viewed by others
Availability of data and material
The data that support the findings of this study are available from the corresponding author, upon reasonable request.
References
Moore SW (2017) Genetic impact on the treatment & management of Hirschsprung disease. J Pediatr Surg 52(2):218–222. https://doi.org/10.1016/j.jpedsurg.2016.11.012
Jaroy EG, Acosta-Jimenez L, Hotta R et al (2019) “Too much guts and not enough brains”: (epi)genetic mechanisms and future therapies of Hirschsprung disease - a review. Clin Epigenetics 11(1):135. https://doi.org/10.1186/s13148-019-0718-x
Bahrami A, Joodi M, Moetamani-Ahmadi M et al (2018) Genetic background of hirschsprung disease: a bridge between basic science and clinical application. J Cell Biochem 119(1):28–33. https://doi.org/10.1002/jcb.26149
Shin TJ, Lee KH, Cho JY (2020) Epigenetic mechanisms of LncRNAs binding to protein in carcinogenesis. Cancers (Basel) 12(10). https://doi.org/10.3390/cancers12102925
Chen S, Shen X (2020) Long noncoding RNAs: functions and mechanisms in colon cancer. Mol Cancer 19(1):167. https://doi.org/10.1186/s12943-020-01287-2
Cai Y, Wan J (2018) Competing endogenous RNA regulations in neurodegenerative disorders: current challenges and emerging insights. Front Mol Neurosci 11:370. https://doi.org/10.3389/fnmol.2018.00370
Sharma U, Barwal TS, Khandelwal A et al (2021) LncRNA ZFAS1 inhibits triple-negative breast cancer by targeting STAT3. Biochimie 182:99–107. https://doi.org/10.1016/j.biochi.2020.12.026
Fan G, Jiao J, Shen F et al (2020) Upregulation of lncRNA ZFAS1 promotes lung adenocarcinoma progression by sponging miR-1271-5p and upregulating FRS2. Thoracic cancer 11(8):2178–2187. https://doi.org/10.1111/1759-7714.13525
Wang JS, Liu QH, Cheng XH et al (2018) The long noncoding RNA ZFAS1 facilitates bladder cancer tumorigenesis by sponging miR-329. Biomed Pharmacother 103:174–181. https://doi.org/10.1016/j.biopha.2018.04.031
Hu F, Shao L, Zhang J et al (2020) Knockdown of ZFAS1 inhibits hippocampal neurons apoptosis and autophagy by activating the PI3K/AKT pathway via up-regulating miR-421 in epilepsy. Neurochem Res 45(10):2433–2441. https://doi.org/10.1007/s11064-020-03103-1
Han CL, Ge M, Liu YP et al (2018) Long non-coding RNA H19 contributes to apoptosis of hippocampal neurons by inhibiting let-7b in a rat model of temporal lobe epilepsy. Cell Death Dis 9(6):617. https://doi.org/10.1038/s41419-018-0496-y
Sergi CM, Caluseriu O, McColl H et al (2017) Hirschsprung’s disease: clinical dysmorphology, genes, micro-RNAs, and future perspectives. Pediatr Res 81(1–2):177–191. https://doi.org/10.1038/pr.2016.202
Heanue TA, Pachnis V (2007) Enteric nervous system development and Hirschsprung’s disease: advances in genetic and stem cell studies. Nat Rev Neurosci 8(6):466–479. https://doi.org/10.1038/nrn2137
Chng SH, Pachnis V (2020) Enteric nervous system: lessons from neurogenesis for reverse engineering and disease modelling and treatment. Curr Opin Pharm 50:100–106. https://doi.org/10.1016/j.coph.2020.02.001
Feng J, Zhou Z, Feng R et al (2021) Silencing long non-coding RNA zinc finger antisense 1 restricts secondary cerebral edema and neuron injuries after traumatic brain injury. Neurosci Lett 756:135958. https://doi.org/10.1016/j.neulet.2021.135958
Stanchina L, Baral V, Robert F et al (2006) Interactions between Sox10, Edn3 and Ednrb during enteric nervous system and melanocyte development. Dev Biol 295(1):232–249. https://doi.org/10.1016/j.ydbio.2006.03.031
Chen F, Zheng H, Zhang W et al (2021) circ_0003170 aggravates human hippocampal neuron injuries by regulating the miR-421/CCL2 axis in cells models of epilepsy. Gen Physiol Biophys 40(2):115–126. https://doi.org/10.4149/gpb_2020045
Li Y, Fang J, Zhou Z et al (2020) Downregulation of lncRNA BACE1-AS improves dopamine-dependent oxidative stress in rats with Parkinson’s disease by upregulating microRNA-34b-5p and downregulating BACE1. Cell Cycle 19(10):1158–1171. https://doi.org/10.1080/15384101.2020.1749447
Lai N, Wu D, Liang T et al (2020) Systemic exosomal miR-193b-3p delivery attenuates neuroinflammation in early brain injury after subarachnoid hemorrhage in mice. J Neuroinflammation 17(1):74. https://doi.org/10.1186/s12974-020-01745-0
von Mering C, Huynen M, Jaeggi D et al (2003) STRING: a database of predicted functional associations between proteins. Nucleic Acids Res 31(1):258–261. https://doi.org/10.1093/nar/gkg034
Amir H, Touboul T, Sabatini K et al (2017) Spontaneous single-copy loss of TP53 in human embryonic stem cells markedly increases cell proliferation and survival. Stem Cells 35(4):872–885. https://doi.org/10.1002/stem.2550
Oh L, Hafsi H, Hainaut P et al (2019) p53, stem cell biology and childhood blastomas. Curr Opin Oncol 31(2):84–91. https://doi.org/10.1097/CCO.0000000000000504
Ge J, Lin H, Yang J et al (2021) TP53-induced glycolysis and apoptosis regulator (TIGAR) ameliorates lysosomal damage in the 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine-mediated mouse model of Parkinson’s disease. Toxicol Lett 339:60–69. https://doi.org/10.1016/j.toxlet.2020.12.011
Gaidin SG, Turovskaya MV, Gavrish MS et al (2020) The selective BDNF overexpression in neurons protects neuroglial networks against OGD and glutamate-induced excitotoxicity. Int J Neurosci 130(4):363–383. https://doi.org/10.1080/00207454.2019.1691205
Acknowledgements
This research project was supported by the Science Foundation of Suzhou Science and Technology Bureau (grant no. SYS2020158).
Funding
This study was supported by the Natural Science Foundation of China (NSFC 82100534) and the Science Foundation of Suzhou Science and Technology Bureau (SYS 2020158).
Author information
Authors and Affiliations
Contributions
All the authors contributed to the study conception and design, data collection and analysis. The first draft of the manuscript was written by Y. W., and revised by P. C. and J. W. All the authors approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
This study was approved by the Ethics Committee of Children’s Hospital of Soochow University, and conducted in compliance with the principles of the Helsinki Declaration.
Consent to participate
Informed consent was obtained from the legal guardians of each child included in the study.
Consent for publication
Informed consent regarding data publishing was obtained from the legal guardians of each child.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, Y., Cai, P. & Wang, J. Clinical significance and biological effect of ZFAS1 in Hirschsprung’s disease and preliminary exploration of its underlying mechanisms using integrated bioinformatics analysis. Ir J Med Sci 191, 2669–2675 (2022). https://doi.org/10.1007/s11845-021-02906-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11845-021-02906-7