Abstract
The conditions experienced by one plant generation can influence the growth of the offspring generation. These maternal effects can reduce performance of foliar-feeding insects, through accumulation of plant defences. Arbuscular mycorrhizal fungi (AMF) inhabit the roots of plants and are known to influence the performance of foliar-feeding insects. However, all published studies of the interactions between insects and AMF have taken place within one plant generation, but none across generations. Thus, in the present study, Senecio vulgaris plants were grown with or without aphids and AMF (termed ‘induction events’), and seeds from each treatment were used to grow plants experiencing that same treatment over four successive generations, all grown in identical environmental conditions. Naïve aphids were reared on Senecio plants whose parents had experienced 0, 1, 2 or 3 induction events. We found strong negative maternal effects of herbivory on aphid growth, which were not mitigated by the mycorrhiza. However, teneral weight and growth rate showed a gradual recovery; aphids reared on plants whose previous three generations suffered attack were similar in size to those at the beginning of the study. Herbivory had positive or negative effects on the mycorrhiza, dependent upon the number of previous generations suffering attack or having mycorrhizal associations. We conclude that the outcome of many insect plant fungal experiments is likely to have been influenced by and need to account for maternal effects of the parental plant’s growth conditions.
Similar content being viewed by others
Introduction
An increasing body of evidence has shown that changes in plant resistance induced by insect herbivores can persist across plant generations, with consequences for herbivores attacking those subsequent cohorts of plants (Rasmann et al. 2012; Neylan et al. 2018; Kafle and Wurst 2019). These effects can be mediated by plant morphology (Agrawal 2001), chemical defences in seeds or vegetative parts (Rasmann et al. 2012; Ballhorn et al. 2016; Dong et al. 2017), or by foliar endophytic fungi (Gundel et al. 2017). Such effects are thought to be adaptive when the herbivory levels experienced by progeny are similar to those of the maternal plants (Herman and Sultan 2011).
Within any one generation, any plant attacked by an insect is likely to be simultaneously colonised or infected by an array of microbial species, including mycorrhizal fungi, endophytes, and pathogens (Hartley and Gange 2009; Fernandez-Conradi et al. 2018). Arbuscular mycorrhizal fungi have received much attention and there appears to be a general pattern whereby generalist chewing insects are negatively affected by mycorrhizal presence, whilst specialist chewers and suckers are positively affected (Koricheva et al. 2009). However, in many cases, there is a continuum of insect responses, from positive to negative, mediated by many factors including soil nutrient status, plant age, plant genetics, fungal identity, and extent of colonisation of the root system (Gange 2007; Rasmussen et al. 2017; Tomczak and Müller 2017). One hitherto overlooked feature is whether the previous generation of plants had mycorrhizal associations and if they were attacked by the insect in question. Experiments investigating maternal effects involving arbuscular mycorrhizal fungi (AMF) are few, but there is some evidence that fungal colonisation of parent plants affects offspring vigour in the next generation, mostly via enhanced nutrient content of seeds (Koide and Lu 1995; Varga et al. 2013). However, to date, experiments across plant generations involving insects and plant-associated beneficial fungi have not been conducted to our knowledge (Gundel et al. 2017). Furthermore, the production of at least one generation of plants before any experiment begins is important to control for any effects of the maternal plant environment (Latzel 2015), and yet is hardly ever practised in insect-mycorrhizal research.
A continuum of responses has also been observed in experiments examining the effects of insect herbivory on the extent of mycorrhizal colonisation of host plants (Barto and Rillig 2010). The carbon-limitation hypothesis suggests that photosynthetic tissue removal by insect herbivores will result in reduced fungal colonisation, as carbon that might have been allocated to the mycorrhiza is directed towards other processes, such as growth and/or defence (Gehring and Whitham 2002). Such an effect has been seen in some annual plants (Barto and Rillig 2010), but is again determined by many factors, including environmental conditions, plant and fungus identity, and herbivore feeding mode (Gange et al. 2002; Gehring and Bennett 2009). Whilst foliar chewing insects often exert negative effects on mycorrhizal fungi (Gange 2007), sucking insects such as aphids may exert negative or positive effects, again dependent on environmental conditions (Babikova et al. 2014; Charters et al. 2020).
Myzus persicae (Sulzer) is an example of a generalist aphid that has shown positive, null or negative responses to mycorrhizal colonisation of its host plant (Gange et al. 1999a; Wurst et al. 2004; Tomczak and Müller 2017). It is frequently found on Senecio vulgaris L. (Asteraceae) in the UK, and can occur on this host throughout the year, as the plant can grow as a summer or winter annual (Heathcote and Byford 1975). The performance of M. persicae on any given host plant is also influenced by the identity of the plant species that the previous generation fed upon (Olivares-Donoso et al. 2007). Senecio vulgaris forms relatively low levels of arbuscular mycorrhizal colonisation (West 1995; Gange et al. 1999b), and whilst morphological effects, such as changes in leaf number have been found in some experiments, the fungi clearly interact with the plant, as their presence dramatically reduces foliar infection by the rust fungus, Puccinia lagenophorae Cooke (West 1995). Moreover, there is some evidence of maternal effects in this plant, as mycorrhizal colonisation of maternal plants can increase or decrease offspring reproductive capacity (though not leaf number), dependent upon soil nutrient status (West 1995).
Maternal effects on offspring performance of S. vulgaris were also reported by Aarssen and Burton (1990). These were also dependent upon soil nutrient levels, with offspring (all grown in a standard fertile soil) being smaller when maternal plants had experienced impoverished soil nutrients. This plant is ideal for studies of maternal effects, as it readily self-pollinates, grows relatively quickly and sets large amounts of viable seed, even when generations are continuously selfed (Aarssen and Burton 1990; Walter et al. 2020). Furthermore, use of the nonradiate flower morph virtually guarantees selfing, as the outcrossing frequency is negligible (Aarssen and Burton 1990).
In this study, our objective was to determine if interactions between aphids and mycorrhizal fungi occurred across plant generations. Over the course of 3 years, we raised five generations of S. vulgaris in constant environmental conditions by selfing and over the latter four generations, conducted factorial experiments involving treatments with and without aphids (M. persicae) on plants that were or were not inoculated with a mixture of mycorrhizal fungal species (see “Materials and methods” section). We asked whether aphid growth changed over the generations, and whether this was influenced by mycorrhizal colonisation. Furthermore, we examined the reciprocal interactions of aphid herbivory on mycorrhizal colonisation. We hypothesised that aphid growth would decrease on plants with and without mycorrhizal fungi, grown from mother plants which experienced aphid herbivory. This would be due to maternal transmission of induced defences, but we also predicted that this would attenuate, due to there being limits on defence production. We further hypothesised that any effects of previous herbivory would be negated by the presence of the mycorrhiza due to the enhanced nutrients that the fungus provides (West 1995). Finally, we hypothesised that aphid attack in any generation would reduce colonisation by the mycorrhizal fungi.
Materials and methods
Plant growth conditions and parameters measured
Senecio vulgaris seeds were collected from over 50 nonradiate flower morph wild plants growing near Salisbury Plain, Wiltshire, UK (grid reference SU196399) in autumn 2014. These seeds were planted into identical individual pots containing 165 g of John Innes grade 3 compost (Westland Horticulture, Huntingdon, UK), so that soil quality remained consistent across the plant generations. The plants were grown in controlled conditions (20 °C, 78% RH and 16 h daylight). The pots were arranged randomly on workbenches and were rearranged once a week to try and reduce any effects of environmental variation. Plants from the wild seeds were grown without mycorrhizal inoculum or insect herbivores, until they produced seeds, which were collected for use in the main experiment. This was done to satisfy the conditions of at least one generation of plants being produced before any experiment took place (Latzel 2015).
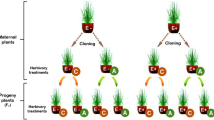
For all subsequent generations, plants were grown individually in pots in an identical manner to those in the pre-experiment generation (above). There were four treatments with 20 plants in each treatment per generation, wherein plants were grown with or without aphids and with or without arbuscular mycorrhizal fungi, in a factorial design, for four generations (Fig. S1). Mycorrhizal plants received 5 ml of ‘Rootgrow’ (PlantWorks Ltd, Sittingbourne, Kent, UK, containing Claroideoglomus claroideum, Funneliformis geosporus, F. mosseae, Glomus microaggregatum, and Rhizophagus irregularis) mixed species inoculum added 2 cm below the topsoil of each pot before the seeds were sown. Non-mycorrhizal plants received an identical amount of autoclaved inoculum, together with a microbial wash to correct for the non-AMF community. The plants were given no supplementary fertiliser and were watered every 3 days with 15 ml tap water each. Once the plants started to set seed, seeds were collected from each plant, pooled within each of the four treatments, and stored at 4 °C in paper envelopes. As many hundreds of seeds were collected from the twenty plants in each treatment at the end of each induction, it is extremely unlikely that the seeds used in the following generation came from just one or a few plants. At the end of each generation, which took roughly 90 days from germination, the plants in each treatment were harvested and total leaf number counted, as a measure of growth. The seeds from experimental plants in each treatment were used to produce the next generation experiencing that same treatment. As each experiment involved successive insect herbivory events or AMF colonisation, we hereafter refer to these as ‘inductions’, following the terminology of Neylan et al. (2018). Thus, there were three induction events over the four plant generations (Fig. S1).
It was logistically impossible to measure seed nitrogen content of every plant in each treatment over all generations. Thus, over 200 seeds from the twenty plants in each treatment within each generation were pooled and random sub samples taken for analysis. The number of seeds varied as the tests relied upon weight and not number. Seeds were oven dried at 60 °C for 48 h, ground to a fine powder and 10 mg weighed into tin capsules (CE instruments, Wigan, UK) and sealed. Nitrogen content was measured using combustion-gas chromatography with an NC soil analyser flash EA 1112 series with a CHNS configuration. The sample was introduced by an autosampler connected to a quartz reactor in a furnace at a temperature of 900 °C. Each sample was burnt and the NO2 from oxidation was transported in a carrier gas (helium), separated by the gas chromatography column and N2 measured by the thermal conductivity detector. There were quality controls (Sulphanilamide STD) (CE instruments, Wigan, UK) with known nitrogen concentrations added to the autosampler throughout the sample run. The nitrogen concentrations of the quality controls were checked against the standards to ensure the results were not drifting through any sample run.
Aphid growth parameters
Myzus persicae were obtained from Rothamsted Research, Harpenden, UK. They were kept in a large insect cage in controlled conditions (20 °C and 16 h daylight) and were reared continuously upon non-flowering Chinese cabbage (Brassica rapa subsp. chinensis L.). This was so the aphids used in each experiment were naïve and maternal effects in the insects were not confused with maternal effects in the plants. Cabbage plants were grown from seeds (Premier Seeds Direct, Salisbury, UK) planted in John Innes grade 3 compost. The aphid colony was continuously re-started with new plants grown in identical conditions, so as to prevent numbers increasing and winged morphs being produced.
To avoid complete plant death through rapid aphid population build-up, each experiment was started with aphids taken from the lab colony once the S. vulgaris plants had become mature and begun bud production. The method was adapted from Leather and Dixon (1984). Three apterous adult M. persicae from the aphid colony were placed onto a mature leaf on each plant. The aphids were unable to move from the leaf due to ‘Oecotak’ barrier glue (Oecos Ltd, Kimpton, UK) being placed around the petiole of each leaf. The aphids were left on the leaves until they had produced nymphs, which could take up to 3 days. Once nymphs were produced, the adults were removed from the plant. The nymphs were weighed using a Sartorius MSP microbalance (accuracy to 0.002 mg) and placed back onto the specific leaf and left until they reached the teneral (recently moulted, but pre-reproductive) adult life stage. These adults were removed from the leaf, weighed together and placed back onto the same leaf to begin reproduction. The weights were averaged by dividing the weight by the number of adult aphids that were weighed. When the adults began to produce nymphs, these were counted daily and removed from the plant, to reduce overcrowding. The plants were checked daily for new nymphs until the adult stopped reproducing or died. The leaf used was in a similar position and of a similar age in each plant generation.
Aphid weight differences between the life stages of nymph and teneral adult were recorded to calculate the mean relative growth rate, using the equation given by Leather and Dixon (1984). To calculate the intrinsic rate of population increase (rm), the time taken to reach adulthood and produce the first nymph was recorded, after which the number of nymphs produced over a period equivalent to this time was measured. The rate of increase was then calculated with the formula given in Wyatt and White (1977).
Visualisation of arbuscular mycorrhizal fungi
The roots were collected from each plant and stored in 70% ethanol until they were ready to be analysed. A random sample of five plants in the non-mycorrhizal treatments for each generation was also checked for mycorrhizal colonisation. For visualization, roots were washed in tap water to remove any visible signs of soil. Roots were placed into labelled square mesh tissue cassettes (Thermos Fisher Scientific, Waltham, USA) and cleared in 10% potassium hydroxide (KOH) in a water bath at 75 °C for 10 min. The KOH was discarded and the roots were thoroughly washed with tap water. A modified ink staining method (Vierheilig et al. 1998) was used to visualise the AMF. Parker washable Quink (Newhaven, UK) was mixed with 1% hydrochloric acid (HCl) and distilled water in the ratio 0.6:15:84.4. Cleaned root samples were added to the stain in a heated water bath at 75 °C for 15 min. The stain was discarded and the root samples were mounted onto slides. The slides had distilled water added to prevent the roots from drying out and the coverslip was sealed with clear nail varnish.
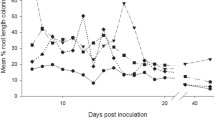
Total percentage root length colonised was obtained with the cross-hair eyepiece method (McGonigle et al. 1990). Roots were spread evenly across the slide and observed at × 200 magnification. Each root piece at the centre of the eyepiece (cross-hair) was observed for presence and absence of fungal features (hyphae, vesicles and arbuscules) and recorded. Approximately 100 views were counted for each root sample.
Statistical analysis
Analyses of aphid weight, growth rate and intrinsic rate of increase, and plant parameters were performed in R 4.0.5 (R Core Team 2018). Normality tests were performed on whole data sets and data were transformed if necessary using lambda calculated by Box–Cox transformation. Differences in the aphid growth parameters over inductions were examined using a two factor ANOVA, employing AMF presence/absence and induction event as the main effects. Differences in percent root length colonised were examined using a two factor ANOVA, employing aphid presence/absence and induction event as the main effects. Colonisation data were subjected to the logit transformation prior to analysis (Warton and Hui 2011). Plant growth parameters (leaf number and seed N content) were subjected to three factor ANOVA, employing plant generation, AMF presence/absence and aphid presence/absence as the main effects. Stepwise deletion was performed to determine the minimal adequate model in each case, with non-significant interaction terms dropped.
Results
Aphid parameters
Aphids reared on the first generation of both non-mycorrhizal and mycorrhizal plants (i.e. whose parents had not been subject to herbivory nor were mycorrhizal, i.e. no inductions) were considerably heavier than those in which parent plants had experienced one or two induction events (F3,152 = 5.08, P = 0.0022) (Fig. 1). Aphid weight on both mycorrhizal and non-mycorrhizal plants appeared to recover with successive inductions and the effect disappeared when aphids fed upon plants whose parents had been attacked in three previous generations (three inductions). Overall, mycorrhizal colonisation had no effect on teneral weight of aphids raised upon S. vulgaris, however, aphids reared on mycorrhizal plants were smaller on plants whose parents had experienced one induction event (F1,30 = 6.07, P = 0.0197) (Fig. 1).
Mean teneral weight of M. persicae when reared on plants with or without arbuscular mycorrhizal fungi and whose parents had not been attacked by aphids (no inductions) or which had experienced one, two or three successive generations of attack (herbivore induction events). Vertical lines represent ± one standard error
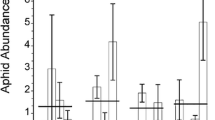
A similar, but weaker pattern of decline over plant generations was seen in aphid mean relative growth rate (F3,152 = 3.38, P = 0.02). Here, the pattern was more variable, with aphids feeding on plants whose parents had been induced twice having similar growth rates to those on plants with no inductions (Fig. 2). Mycorrhizal colonisation had no effect on growth rate in any plant generation. Aphid intrinsic rate of increase also showed a large decrease after one induction event (F3,152 = 8.02, P < 0.001), with no further pattern of change. Unlike teneral weight and growth rate, there appeared to be no recovery in this parameter after three induction events (Fig. 3). Mycorrhizal colonisation again had no effect on intrinsic rate of population increase of aphids in any generation. In all plant generations, aphids on mycorrhizal plants responded similarly to those on non-mycorrhizal plants, leading to an absence of any interaction terms between induction event number and mycorrhizal presence in any generation.
Mean relative growth rate of M. persicae when reared on plants with or without arbuscular mycorrhizal fungi and whose parents had not been attacked by aphids (no inductions) or which had experienced one, two or three successive generations of attack (herbivore induction events). Vertical lines represent ± one standard error
Mean intrinsic rate of increase of M. persicae when reared on plants with or without arbuscular mycorrhizal fungi and whose parents had not been attacked by aphids (no inductions) or which had experienced one, two or three successive generations of attack (herbivore induction events). Vertical lines represent ± one standard error
Mycorrhizal colonisation
There was no significant effect of aphid herbivory on mycorrhizal colonisation across the inductions. However, the pattern varied greatly, depending on the number of induction events (Fig. 4). Aphid attack increased colonisation if no inductions had occurred but dramatically decreased it after two inductions. Meanwhile, there was no difference after three inductions. These inconsistencies were reflected in a significant interaction term between induction event number and aphid attack (F3,152 = 4.12, P = 0.0253). No mycorrhizal colonisation was seen in the control plant roots in any of the plant generations.
Mean percent root length colonised by mycorrhizal fungi of plants with or without aphids and whose parents had not been colonised by arbuscular mycorrhizal fungi (no inductions) or which had experienced one, two or three successive generations of colonisation (fungal induction events). Vertical lines represent ± one standard error
Plant growth
Across generations and treatments, those plants that grew following two or three induction events (i.e. in the third and fourth generations) were considerably larger and bore more leaves than plants in the first two generations (F3,304 = 35.22, P < 0.001) (Fig. 5). This phenomenon was most pronounced after the third induction event, in the absence of both aphids and mycorrhizal fungi, suggesting a clear maternal effect in this plant species. Aphid attack reduced leaf number if no inductions had occurred (F1,76 = 20.91, P < 0.001), however, this effect disappeared after one, two and three inductions. There was a suggestion that mycorrhizal fungi alone reduced leaf number after three inductions compared to those treatments involving aphids, but overall, the fungi had no effect on size, nor any interactions with aphid attack (Fig. 5). Although no significant interactions were found, there was a trend of plants in the aphids and mycorrhizal fungal treatment to bear more leaves with each successive induction (Fig. 5).
Across generations, those plants that grew following one or two inductions produced seeds that had lower nitrogen contents (F3,64 = 6.05, P = 0.0019) than the control plants. However, this effect was only seen in plants that had been attacked by aphids (Fig. 6), leading to a significant interaction term between induction event number and insect attack (F3,64 = 24.43, P < 0.001). Although there was no overall effect of mycorrhizal colonisation on seed N, levels in the combined treatment recovered much more than in the aphid treatment alone, leading to a significant three-way interaction term (F3,64 = 7.34, P < 0.001, Fig. 6).
Discussion
It has long been known that plant responses to arbuscular mycorrhizal colonisation are context-dependent, and affected by a wide variety of factors, including plant and fungal identity, soil nutrient status, soil biotic complexity and environmental conditions (Hoeksema et al. 2010). The responses of insect herbivores to mycorrhizal colonisation of their host plants are similarly context-dependent (Barber et al. 2013). Here we show that whether or not the parent plant was attacked by insects or colonised by mycorrhizal fungi is critical in determining the outcome of any experiment. It is important to note that aphids used in each experiment were naïve, all having been reared on cabbage plants. Furthermore, each generation of plants was grown in identical environmental conditions. Thus, the effects we observed must have been due to maternal changes in the quality of the S. vulgaris host plants, and not to any changes in growing conditions or the aphids themselves. Maternal effects in insects can be strong also, and would add further complexity to the outcome of insect-plant-fungal experiments (Moore et al. 2019).
In our study, one herbivory induction event was sufficient to cause a significant reduction in aphid weight, growth rate and intrinsic rate of increase, irrespective of mycorrhizal presence. To an extent, this upheld our first hypothesis, but there was less evidence of attenuation in these parameters than we expected. Indeed, after two or three inductions, weight and growth rate had returned to similar levels seen on plants with no inductions by aphids (Figs. 1, 2). However, intrinsic rate of increase did not recover (Fig. 3). Similar reductions in insect performance have been noted before in both chewing and sucking insects (Ballhorn et al. 2016; Dong et al. 2017) and have been attributed to transgenerational changes in herbivore-induced defences. S. vulgaris synthesises pyrrolizidine alkaloids within the root system which are moved via the phloem for storage in the flower heads and leaves (Hartmann et al. 1989; Cheng et al. 2017; Flade et al. 2019), but it is unknown whether these can be induced by insect damage in this species. In the closely related Jacobaea vulgaris Gaertn. (syn. Senecio jacobaea L.), foliage removal manually or by insects reduced or had no effect on alkaloid levels (van Dam et al. 1993; Hol et al. 2004). Furthermore, the fact that these alkaloids can be easily excreted by M. persicae (Molyneux et al. 1990) suggests that they may have little effect on the performance of this insect. Previous studies have indicated that effects of induced defences on generalist herbivores across plant generations are cumulative (Neylan et al. 2018), so if this was the mechanism, we would not expect the recovery in the performance of this polyphagous aphid that was observed. Our attempts to record alkaloid levels in the S. vulgaris plants failed, as they appeared to be below detectable levels (Chitty 2018), a problem also encountered by Molyneux et al. (1990). Thus, whilst we cannot discount changes in alkaloid plant defences over generations, there is at present relatively little evidence that this occurred. Whilst pyrrolizidine alkaloids are the main defence compounds found in S. vulgaris there are other metabolites that can function as chemical defences. Some of these chemical compounds were only recently obtained from the plant (Andreani et al. 2015) and have not been studied in depth.
Clearly, the medium for transmission of any maternal effects is the seed, and provisioning of nutrients within seeds is thought to be one mechanism by which maternal plants affect offspring defensive phenotypes (Kafle and Wurst 2019). Seed nitrogen content is an important consistent measure of the quality and quantity of parental investment to offspring (Germain et al. 2013) and correlates with leaf and stem N content in other members of the Asteraceae (Nabi et al. 1995). In this study, we found that seed N content was lower in plants with one or two aphid inductions, but had recovered to pre-experimental levels in those with three inductions (Fig. 6). Thus, the observed reduction in aphid growth on plants with one or two inductions and subsequent recovery after three inductions may have been due to changes in food quality. Whilst acknowledging that total N content is a relatively crude measure of food quality for aphids, M. persicae has been shown to have increased fecundity and intrinsic rate of population increase when feeding on fertilised plants compared with non-fertilized plants (Jansson and Smilowitz 1986) and is known to demonstrate a significant preference for plants with higher nutrient content (Van Emden and Bashford 1971). Therefore, it is possible that the effects we observed on aphid growth may perhaps have been determined more by plant nitrogen content, rather than defence accumulation. Further evidence to support this idea may be found in the growth of the plants themselves. Those whose parents had experienced three inductions produced more leaves (Fig. 5), a phenomenon that has been observed before in other plants (Steets and Ashman 2010). Aphid performance on these larger plants, which appeared to contain more nitrogen, may therefore conform to the plant vigour hypothesis, which states that insect herbivores will preferentially choose large, more vigorously growing plants or plant modules because offspring performance will be greater on them (Price 1991).
Arbuscular mycorrhizal fungi may also increase the performance of aphids through delivery of N via the host plant (Wilkinson et al. 2019). However, we did not find any mitigation of herbivory effects by the mycorrhiza, and after one induction, AMF presence appeared to reduce aphid size. Colonisation levels by mycorrhizal fungi were relatively low, but certainly comparable with levels found by previous authors working with S. vulgaris (West 1995; Gange et al. 1999b) and the mycorrhizal interaction with the plant did appear to have some consequences for the aphid. For example, after one induction event, aphid weight on plants from aphid-only parents dropped by 37%, but by 64% when the AMF were present (Fig. 2). Intriguingly, seed N content of the aphid herbivory and AMF colonised plants after one induction showed the lowest seed N content of any induction event, providing further evidence of the role of nitrogen in aphid performance (Fig. 6). However, over all plant generations, AMF had no effect on aphid performance, thus our second hypothesis was not upheld. Two possible explanations may account for the lack of effects. Mycorrhizal colonisation has also been shown to physically enlarge the vascular bundle size in wheat leading to increased aphid fecundity and development (Simon et al. 2017). It is possible that in this study, the colonisation levels could have been too low to have had any effect on plant physiology. More likely is that several studies have shown that inoculation of a single species of AMF seems to affect the insects feeding upon colonised plants, whilst multiple species inocula seem to have less of an effect (Gadhave et al. 2016). We used a mixed inoculum because all plant species in the field are colonised by a number of different fungi simultaneously. It could be that the combination of five different species of AMF used in the mixed inoculum failed to have any effect on the insect, perhaps due to competition between the fungi affecting the carbon-nutrient trade balance and thus sometimes failing to result in significant effects on the plant (Gadhave et al. 2016).
In general, herbivory by aphids can reduce AMF colonisation of plant roots, but such interactions are again context-dependent (Charters et al. 2020). We observed variable effects of herbivory on AMF colonisation, with increased colonisation if parent plants had experienced no or one induction event, but reductions in colonisation after two induction events (Fig. 4). Increases in mycorrhiza colonisation following herbivory are not often recorded and tend to depend on soil nutrient status and the age of the plant (Wamberg et al. 2003). The likely mechanism is the reallocation of carbon to the roots for growth, which may be intercepted by the fungus, or to root exudation, to attract greater fungal colonisation, leading to enhanced nutrient uptake (Gehring and Bennett 2009). Our third hypothesis was thus only partially supported. However, it would seem that the variable responses of mycorrhizal fungi to insect attack that have been previously recorded (Barto and Rillig 2010) could be due to a failure to control for the maternal effects of insects and or mycorrhizal fungi experienced by parents of the seeds used in those experiments.
In conclusion, our results demonstrate that herbivore- and fungal-associated maternal effects in plants are strong. Most previous multi-generational insect-plant experiments record insect growth over one or two generations after an induction (though see Neylan et al. (2018) for an exception). Our results show that the maternal effects observed depend on the number of generations and in some cases insect parameters can apparently recover. We chose to mimic natural field situations, in which it is highly likely for plants in a population of S. vulgaris growing in the same place to be attacked by aphids and to be colonised by mycorrhizal fungi in each succeeding generation. However, S. vulgaris has wind-dispersed seeds, so it is also possible for these to colonise habitats where they escape herbivory or fungal colonisation. Clearly, it would take a series of complex experiments to unravel the possible combinations of induction events. However, such experiments need to be attempted for us to fully understand the complexity of insect plant fungal interactions.
Data availability
All data belonging to this manuscript are available from the corresponding author upon reasonable request.
Code availability
Not applicable.
References
Aarssen LW, Burton SM (1990) Maternal effects at four levels in Senecio vulgaris (Asteraceae) grown on a soil nutrient gradient. Am J Bot 77:1231–1240. https://doi.org/10.2307/2444634
Agrawal AA (2001) Transgenerational consequences of plant responses to herbivory: an adaptive maternal effect? Am Nat 157:555–569. https://doi.org/10.1086/319932
Andreani S, Paolini J, Costa J, Muselli A (2015) Essential-oil composition and chemical variability of Senecio vulgaris L. from Corsica. Chem Biodivers 12:752–766. https://doi.org/10.1002/cbdv.201400223
Babikova Z, Gilbert L, Bruce T, Dewhirst SY, Pickett JA, Johnson D (2014) Arbuscular mycorrhizal fungi and aphids interact by changing host plant quality and volatile emission. Funct Ecol 28:375–385. https://doi.org/10.1111/1365-2435.12181
Ballhorn DJ, Kautz S, Laumann JM (2016) Herbivore damage induces a transgenerational increase of cyanogenesis in wild lima bean (Phaseolus lunatus). Chemoecology 26:1–5. https://doi.org/10.1007/s00049-015-0201-x
Barber NA, Kiers ET, Hazzard RV, Adler LS (2013) Context-dependency of arbuscular mycorrhizal fungi on plant-insect interactions in an agroecosystem. Front Plant Sci 4:338. https://doi.org/10.3389/fpls.2013.00338
Barto EK, Rillig MC (2010) Does herbivory really suppress mycorrhiza? A meta-analysis. J Ecol 98:745–753. https://doi.org/10.1111/j.1365-2745.2010.01658.x
Charters MD, Sait SM, Field KJ (2020) Aphid herbivory drives asymmetry in carbon for nutrient exchange between plants and an arbuscular mycorrhizal fungus. Curr Biol 30:1801–1808. https://doi.org/10.1016/j.cub.2020.02.087
Cheng D, Nguyen V, Ndihokubwayo N, Ge J, Mulder PPJ (2017) Pyrrolizidine alkaloid variation in Senecio vulgaris populations from native and invasive ranges. PeerJ 5:e3686. https://doi.org/10.7717/peerj.3686
Chitty RP (2018) Parental effects in Senecio vulgaris. PhD thesis, Royal Holloway, University of London, UK
Dong BC, Fu T, Luo FL, Yu FH (2017) Herbivory-induced maternal effects on growth and defense traits in the clonal species Alternanthera philoxeroides. Sci Total Environ 605:114–123. https://doi.org/10.1016/j.scitotenv.2017.06.141
Fernandez-Conradi P, Jactel H, Robin C, Tack AJM, Castagneyrol B (2018) Fungi reduce preference and performance of insect herbivores on challenged plants. Ecology 99:300–311. https://doi.org/10.1002/ecy.2044
Flade J, Beschow H, Wensch-Dorendorf M, Plescher A, Waetjen W (2019) Occurrence of nine pyrrolizidine alkaloids in Senecio vulgaris L. depending on developmental stage and season. Plants Basel 8:54. https://doi.org/10.3390/plants8030054
Gadhave KR, Hourston JE, Gange AC (2016) Developing soil microbial inoculants for pest management: can one have too much of a good thing? J Chem Ecol 42:348–356. https://doi.org/10.1007/s10886-016-0689-8
Gange AC (2007) Insect-mycorrhizal interactions: patterns, processes and consequences. In: Ohgushi T, Craig TP, Price PW (eds) Ecological communities: plant mediation in interaction webs. Cambridge University Press, Cambridge, pp 124–144
Gange AC, Bower E, Brown VK (1999a) Positive effects of an arbuscular mycorrhizal fungus on aphid life history traits. Oecologia 120:123–131. https://doi.org/10.1007/s004420050840
Gange AC, Bower E, Stagg PG, Aplin DM, Gillam AE, Bracken M (1999b) A comparison of visualization techniques for recording arbuscular mycorrhizal colonization. New Phytol 142:123–132. https://doi.org/10.1046/j.1469-8137.1999.00371.x
Gange AC, Bower E, Brown VK (2002) Differential effects of insect herbivory on arbuscular mycorrhizal colonization. Oecologia 131:103–112. https://doi.org/10.1007/s00442-001-0863-7
Gehring CA, Bennett A (2009) Mycorrhizal fungal-plant-insect interactions: the importance of a community approach. Environ Entomol 38:93–102. https://doi.org/10.1603/022.038.0111
Gehring CA, Whitham TG (2002) Mycorrhizae-herbivore interactions: population and community consequences. In: Van Der Heijden MGA, Sanders IR (eds) Mycorrhizal ecology. Springer, Berlin, pp 295–320
Germain RM, Caruso CM, Maherali H (2013) Mechanisms and consequences of water stress-induced parental effects in an invasive annual grass. Int J Plant Sci 174:886–895. https://doi.org/10.1086/670691
Gundel PE, Rudgers JA, Whitney KD (2017) Vertically transmitted symbionts as mechanisms of transgenerational effects. Am J Bot 104:787–792. https://doi.org/10.3732/ajb.1700036
Hartley SE, Gange AC (2009) Impacts of plant symbiotic fungi on insect herbivores: mutualism in a multitrophic context. Ann Rev Ent 54:323–342. https://doi.org/10.1146/annurev.ento.54.110807.090614
Hartmann T, Ehmke A, Eilert U, Vonborstel K, Theuring C (1989) Sites of synthesis, translocation and accumulation of pyrrolizidine alkaloid N-oxides in Senecio vulgaris L. Planta 177:98–107. https://doi.org/10.1007/bf00392159
Heathcote GD, Byford WJ (1975) Surveys of sugar-beet seed crops, mangold clamps and weeds in England for aphids and viruses, 1963–1973. J Agric Sci 84:87–95. https://doi.org/10.1017/s0021859600071914
Herman JJ, Sultan SE (2011) Adaptive transgenerational plasticity in plants: case studies, mechanisms, and implications for natural populations. Front Plant Sci 2:102. https://doi.org/10.3389/fpls.2011.00102
Hoeksema JD, Chaudhary VB, Gehring CA, Johnson NC, Karst J, Koide RT, Pringle A, Zabinski C, Bever JD, Moore JC, Wilson GWT, Klironomos JN, Umbanhowar J (2010) A meta-analysis of context-dependency in plant response to inoculation with mycorrhizal fungi. Ecol Lett 13:394–407. https://doi.org/10.1111/j.1461-0248.2009.01430.x
Hol WHG, Macel M, van Veen JA, van der Meijden E (2004) Root damage and aboveground herbivory change concentration and composition of pyrrolizidine alkaloids of Senecio jacobaea. Basic Appl Ecol 5:253–260. https://doi.org/10.1016/j.baae.2003.12.002
Jansson RK, Smilowitz Z (1986) Influence of nitrogen on population parameters of potato insects—abundance population growth, and within-plant distribution of the green peach aphid, Myzus persicae (Homoptera, Aphididae). Environ Entomol 15:49–55. https://doi.org/10.1093/ee/15.1.49
Kafle D, Wurst S (2019) Legacy effects of herbivory enhance performance and resistance of progeny plants. J Ecol 107:58–68. https://doi.org/10.1111/1365-2745.13038
Koide RT, Lu X (1995) On the cause of offspring superiority conferred by mycorrhizal infection of Abutilon theophrasti. New Phytol 131:435–441. https://doi.org/10.1111/j.1469-8137.1995.tb03080.x
Koricheva J, Gange AC, Jones T (2009) Effects of mycorrhizal fungi on insect herbivores: a meta-analysis. Ecology 90:2088–2097. https://doi.org/10.1890/08-1555.1
Latzel V (2015) Pitfalls in ecological research—transgenerational effects. Folia Geobot 50:75–85. https://doi.org/10.1007/s12224-015-9208-x
Leather SR, Dixon AFG (1984) Aphid growth and reproductive rates. Entomol Exp Appl 35:137–140. https://doi.org/10.1111/j.1570-7458.1984.tb03373.x
McGonigle TP, Miller MH, Evans DG, Fairchild GL, Swan JA (1990) A new method which gives an objective measure of colonization of roots by vesicular-arbuscular mycorrhizal fungi. New Phytol 115:495–501. https://doi.org/10.1111/j.1469-8137.1990.tb00476.x
Molyneux RJ, Campbell BC, Dreyer DL (1990) Honeydew analysis for detecting phloem transport of plant natural products—implications for host-plant resistance to sap-sucking insects. J Chem Ecol 16:1899–1909. https://doi.org/10.1007/bf01020503
Moore MP, Whiteman HH, Martin RA (2019) A mother’s legacy: the strength of maternal effects in animal populations. Ecol Lett 22:1620–1628. https://doi.org/10.1111/ele.13351
Nabi G, Rahmatullah SM, Gill MA (1995) Partitioning of biomass, N and S in sunflower (Helianthus annuus L.) by nitrogen and sulfur nutrition. J Agron Crop Sci 174:27–32. https://doi.org/10.1111/j.1439-037X.1995.tb00191.x
Neylan IP, Dirzo R, Sobral M (2018) Cumulative effects of transgenerational induction on plant palatability to generalist and specialist herbivores. Web Ecol 18:41–46. https://doi.org/10.5194/we-18-41-2018
Olivares-Donoso R, Troncoso AJ, Tapia DH, Aguilera-Olivares D, Niemeyer HM (2007) Contrasting performances of generalist and specialist Myzus persicae (Hemiptera: Aphididae) reveal differential prevalence of maternal effects after host transfer. Bull Entomol Res 97:61–67. https://doi.org/10.1017/s0007485307004774
Price PW (1991) The plant vigor hypothesis and herbivore attack. Oikos 62:244–251. https://doi.org/10.2307/3545270
R Core Team (2018) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/. Accessed 15th April 2021.
Rasmann S, De Vos M, Casteel CL, Tian DL, Halitschke R, Sun JY, Agrawal AA, Felton GW, Jander G (2012) Herbivory in the previous generation primes plants for enhanced insect resistance. Plant Physiol 158:854–863. https://doi.org/10.1104/pp.111.187831
Rasmussen PU, Amin T, Bennett AE, Karlsson Green K, Timonen S, Van Nouhuys S, Tack AJM (2017) Plant and insect genetic variation mediate the impact of arbuscular mycorrhizal fungi on a natural plant-herbivore interaction. Ecol Entomol 42:793–802. https://doi.org/10.1111/een.12453
Simon AL, Wellham PAD, Aradottir GI, Gange AC (2017) Unravelling mycorrhiza-induced wheat susceptibility to the English grain aphid Sitobion avenae. Sci Rep 7:46497. https://doi.org/10.1038/srep46497
Steets JA, Ashman TL (2010) Maternal effects of herbivory in Impatiens capensis. Int J Plant Sci 171:509–518. https://doi.org/10.1086/651944
Tomczak VV, Müller C (2017) Influence of arbuscular mycorrhizal stage and plant age on the performance of a generalist aphid. J Insect Phys 98:258–266. https://doi.org/10.1016/j.jinsphys.2017.01.016
Van Dam NM, Van der Meijden E, Verpoorte R (1993) Induced responses in three alkaloid-containing plant species. Oecologia 95:425–430. https://doi.org/10.1007/bf00320998
van Emden HF, Bashford MA (1971) The performance of Brevicoryne brassicae and Myzus persicae in relation to plant age and leaf amino acids. Ent Exp Appl 14:349–360. https://doi.org/10.1111/j.1570-7458.1971.tb00172.x
Varga S, Vega-Frutis R, Kytöviita MM (2013) Transgenerational effects of plant sex and arbuscular mycorrhizal symbiosis. New Phytol 199:812–821. https://doi.org/10.1111/nph.12305
Vierheilig H, Coughlan AP, Wyss U, Piché Y (1998) Ink and vinegar, a simple staining technique for arbuscular mycorrhizal fungi. Appl Environ Microbiol 64:5004–5007. https://doi.org/10.1128/AEM.64.12.5004-5007.1998
Walter GM, Abbott RJ, Brennan AC, Bridle JR, Chapman M, Clark J, Filatov D, Nevado B, Ortiz-Barrientos D, Hiscock SJ (2020) Senecio as a model system for integrating studies of genotype, phenotype and fitness. New Phytol 226:326–344. https://doi.org/10.1111/nph.16434
Wamberg C, Christensen S, Jakobsen I (2003) Interaction between foliar-feeding insects, mycorrhizal fungi, and rhizosphere protozoa on pea plants. Pedobiologia 47:281–287. https://doi.org/10.1078/0031-4056-00191
Warton DI, Hui FKC (2011) The arcsine is asinine: the analysis of proportions in ecology. Ecology 92:3–10. https://doi.org/10.1890/10-0340.1
West HM (1995) Soil phosphate status modifies response of mycorrhizal and nonmycorrhizal Senecio vulgaris L. to infection by the rust, Puccinia lagenophorae Cooke. New Phytol 129:107–116. https://doi.org/10.1111/j.1469-8137.1995.tb03014.x
Wilkinson TDJ, Ferrari J, Hartley SE, Hodge A (2019) Aphids can acquire the nitrogen delivered to plants by arbuscular mycorrhizal fungi. Funct Ecol 33:576–586. https://doi.org/10.1111/1365-2435.13283
Wurst S, Dugassa-Gobena D, Langel R, Bonkowski M, Scheu S (2004) Combined effects of earthworms and vesicular-arbuscular mycorrhizas on plant and aphid performance. New Phytol 163:169–176. https://doi.org/10.1111/j.1469-8137.2004.01106.x
Wyatt IJ, White PF (1977) Simple estimation of intrinsic increase rates for aphids and tetranychid mites. J Appl Ecol 14:757–766. https://doi.org/10.2307/2402807
Acknowledgements
We are grateful to those who helped to collect the initial plants, including Maria Hine and the Chitty family. We also thank José Valcarcel (Department of Geography, Royal Holloway) for assisting with the soil chemistry work and Jack Forster at Forest Research for statistical advice.
Funding
This research was funded by Royal Holloway, University of London.
Author information
Authors and Affiliations
Contributions
RPC and ACG designed the study, which was executed and analysed by RPC. Both authors contributed to the writing of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Handling Editor: Kerry Mauck.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chitty, R.P., Gange, A.C. Reciprocal interactions between aphids and arbuscular mycorrhizal fungi across plant generations. Arthropod-Plant Interactions 16, 33–43 (2022). https://doi.org/10.1007/s11829-021-09875-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11829-021-09875-9