Abstract
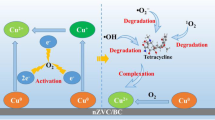
Polydopamine/NZVI@biochar composite (PDA/NZVI@BC) with high removal efficiency of tetracycline (TC) in aqueous solutions was successfully synthesized. The resultant composite demonstrated high reactivity, excellent stability and reusability over the reaction course. Such excellent performance can be attributed to the presence of the huge surface area on biochar (BC), which could enhance NZVI dispersion and prolong its longevity. The carbonyl group contained on the surface of biochar could combine with the amino group on polydopamine(PDA). The hydroxyl groups in PDA is able to enhance the dispersion and loading of NZVI on BC. Being modified by PDA, the hydrophilicity of biochar was improved. Among BC, pristine NZVI and PDA/NZVI@BC, PDA/ NZVI@BC exhibited the highest activity for removal of TC. Compared with NZVI, the removal efficiency of TC could be increased by 55.9% by using PDA/NZVI@BC under the same conditions. The optimal modification time of PDA was 8h, and the ratio of NZVI to BC was 1:2. In addition, the possible degradation mechanism of TC was proposed, which was based on the analysis of degraded products by LC-MS. Different important factors impacting on TC removal (including mass ratio of NZVI to BC/PDA, initial concentration, pH value and the initial temperature of the solution) were investigated as well. Overall, this study provides a promising alternative material and environmental pollution management option for antibiotic wastewater treatment.

Similar content being viewed by others
References
An B, Liang Q, Zhao D (2011). Removal of arsenic(V) from spent ion exchange brine using a new class of starch-bridged magnetite nanoparticles. Water Research, 45(5): 1961–1972
Arshadi M, Abdolmaleki M K, Mousavinia F, Foroughifard S, Karimzadeh A (2017). Nano modification of NZVI with an aquatic plant Azolla filiculoides to remove Pb(II) and Hg(II) from water: Aging time and mechanism study. Journal of Colloid and Interface Science, 486: 296–308
Arshadi M, Soleymanzadeh M, Salvacion J W, SalimiVahid F (2014). Nanoscale Zero-Valent Iron (NZVI) supported on sineguelas waste for Pb(II) removal from aqueous solution: Kinetics, thermodynamic and mechanism. Journal of Colloid and Interface Science, 426: 241–251
Cai Z, Fu J, Du P, Zhao X, Hao X, Liu W, Zhao D (2018). Reduction of nitrobenzene in aqueous and soil phases using carboxymethyl cellulose stabilized zero-valent iron nanoparticles. Chemical Engineering Journal, 332: 227–236
Cao M, Wang L, Ai Z, Zhang L (2015). Efficient remediation of pentachlorophenol contaminated soil with tetrapolyphosphate washing and subsequent ZVI/Air treatment. Journal of Hazardous Materials, 292: 27–33
Chen S S, Hsu B C, Hung LW (2008). Chromate reduction by waste iron from electroplating wastewater using plug flow reactor. Journal of Hazardous Materials, 152(3): 1092–1097
Chen WR, Huang C H (2009). Transformation of tetracyclines mediated by Mn(II) and Cu(II) ions in the presence of oxygen. Environmental Science & Technology, 43(2): 401–407
Daghrir R, Drogui P (2013). Tetracycline antibiotics in the environment: A review. Environmental Chemistry Letters, 11(3): 209–227
Ding Y H, Floren M, Tan W (2016). Mussel-inspired polydopamine for bio-surface functionalization. Biosurface and Biotribology, 2(4): 121–136
Dong H, Deng J, Xie Y, Zhang C, Jiang Z, Cheng Y, Hou K, Zeng G (2017). Stabilization of nanoscale zero-valent iron (nZVI) with modified biochar for Cr(VI) removal from aqueous solution. Journal of Hazardous Materials, 332: 79–86
Dong H, Xie Y, Zeng G, Tang L, Liang J, He Q, Zhao F, Zeng Y, Wu Y (2016). The dual effects of carboxymethyl cellulose on the colloidal stability and toxicity of nanoscale zero-valent iron. Chemosphere, 144: 1682–1689
Feng J, Zhu B W, Lim T T (2008). Reduction of chlorinated methanes with nano-scale Fe particles: Effects of amphiphiles on the dechlorination reaction and two-parameter regression for kinetic prediction. Chemosphere, 73(11): 1817–1823
Ghauch A, Tuqan A, Assi H A (2009). Antibiotic removal from water: elimination of amoxicillin and ampicillin by microscale and nanoscale iron particles. Environmental Pollution, 157(5): 1626–1635
Guan X, Sun Y, Qin H, Li J, Lo I M, He D, Dong H (2015). The limitations of applying zero-valent iron technology in contaminants sequestration and the corresponding countermeasures: The development in zero-valent iron technology in the last two decades (1994–2014). Water Research, 75: 224–248
He F, Zhao D (2008). Hydrodechlorination of trichloroethene using stabilized Fe-Pd nanoparticles: Reaction mechanism and effects of stabilizers, catalysts and reaction conditions. Applied Catalysis B: Environmental, 84(3-4): 533–540
Hsieh W P, Pan J R, Huang C, Su Y C, Juang Y J (2010). Enhance the photocatalytic activity for the degradation of organic contaminants in water by incorporating TiO2 with zero-valent iron. Science of the Total Environment, 408(3): 672–679
Jeong J, Song W, Cooper W J, Jung J, Greaves J (2010). Degradation of tetracycline antibiotics: Mechanisms and kinetic studies for advanced oxidation/reduction processes. Chemosphere, 78(5): 533–540
Jiang J, Zhu L, Zhu L, Zhang H, Zhu B, Xu Y (2013). Antifouling and antimicrobial polymer membranes based on bioinspired polydopamine and strong hydrogen-bonded poly(N-vinyl pyrrolidone). ACS Applied Materials & Interfaces, 5(24): 12895–12904
Lee H, Dellatore S M, Miller W M, Messersmith P B (2007). Musselinspired surface chemistry for multifunctional coatings. Science, 318 (5849): 426–430
Li J, Bao H, Xiong X, Sun Y, Guan X (2015a). Effective Sb(V) immobilization from water by zero-valent iron with weak magnetic field. Separation and Purification Technology, 151: 276–283
Li R, Jin X, Megharaj M, Naidu R, Chen Z (2015b). Heterogeneous Fenton oxidation of 2,4-dichlorophenol using iron-based nanoparticles and persulfate system. Chemical Engineering Journal, 264: 587–594
Li Y, Cheng W, Sheng G, Li J, Dong H, Chen Y, Zhu L (2015c). Synergetic effect of a pillared bentonite support on Se(VI) removal by nanoscale zero valent iron. Applied Catalysis B: Environmental, 174–175: 329–335
Loget G, Yoo J E, Mazare A, Wang L, Schmuki P (2015). Highly controlled coating of biomimetic polydopamine in TiO2 nanotubes. Electrochemistry Communications, 52: 41–44
Lyu H, Zhao H, Tang J, Gong Y, Huang Y, Wu Q, Gao B (2018). Immobilization of hexavalent chromium in contaminated soils using biochar supported nanoscale iron sulfide composite. Chemosphere, 194: 360–369
Rodriguez-Mozaz S, Chamorro S, Marti E, Huerta B, Gros M, Sànchez-Melsió A, Borrego C M, Barceló D, Balcázar J L (2015). Occurrence of antibiotics and antibiotic resistance genes in hospital and urban wastewaters and their impact on the receiving river. Water Research, 69: 234–242
Sarmah A K, Meyer M T, Boxall A B (2006). A global perspective on the use, sales, exposure pathways, occurrence, fate and effects of veterinary antibiotics (VAs) in the environment. Chemosphere, 65(5): 725–759
Sever M J, Weisser J T, Monahan J, Srinivasan S, Wilker J J (2004). Metal-mediated cross-linking in the generation of a marine-mussel adhesive. Angewandte Chemie, 116: 43(4): 448–450
Shao B, Liu L F, Yang F L, Shan D N, Yuan H (2012). Membrane modification using polydopamine and/or PDA coated TiO2 nano particles for wastewater treatment. Procedia Engineering, 44: 1431–1432
Shen W, Mu Y, Wang B, Ai Z H, Zhang L Z (2017). Enhanced aerobic degradation of 4-chlorophenol with iron-nickel nanoparticles. Applied Surface Science, 393: 316–324
Shi L N, Zhang X, Chen Z L (2011). Removal of chromium (VI) from wastewater using bentonite-supported nanoscale zero-valent iron. Water Research, 45(2): 886–892
Su H, Fang Z, Tsang P E, Fang J, Zhao D (2016b). Stabilisation of nanoscale zero-valent iron with biochar for enhanced transport and in-situ remediation of hexavalent chromium in soil. Environmental Pollution, 214: 94–100
Su H, Fang Z, Tsang P E, Zheng L, Cheng W, Fang J, Zhao D (2016a). Remediation of hexavalent chromium contaminated soil by biocharsupported zero-valent iron nanoparticles. Journal of Hazardous Materials, 318: 533–540
Su J, Lin S, Chen Z, Megharaj M, Naidu R (2011). Dechlorination of pchlorophenol from aqueous solution using bentonite supported Fe/Pd nanoparticles: Synthesis, characterization and kinetics. Desalination, 280(1-3): 167–173
Wang X, Chen C, Liu H, Ma J (2008). Preparation and characterization of PAA/PVDF membrane-immobilized Pd/Fe nanoparticles for dechlorination of trichloroacetic acid. Water Research, 42(18): 4656–4664
Xi Z Y, Xu Y Y, Zhu L P, Wang Y, Zhu B K (2009). A facile method of surface modification for hydrophobic polymer membranes based on the adhesive behavior of poly(DOPA) and poly(dopamine). Journal of Membrane Science, 327(1-2): 244–253
Yang K, Yue Q, Han W, Kong J, Gao B, Zhao P, Duan L (2015). Effect of novel sludge and coal cinder ceramic media in combined anaerobic–aerobic bio-filter for tetracycline wastewater treatment at low temperature. Chemical Engineering Journal, 277: 130–139
Yang L, Phua S L, Teo J K H, Toh C L, Lau S K, Ma J, Lu X (2011). A biomimetic approach to enhancing interfacial interactions: polydopamine-coated clay as reinforcement for epoxy resin. ACS Applied Materials & Interfaces, 3(8): 3026–3032
Yin W, Wu J, Li P, Lin G, Wang X, Zhu B, Yang B (2012a). Reductive transformation of pentachloronitrobenzene by zero-valent iron and mixed anaerobic culture. Chemical Engineering Journal, 210: 309–315
Yin W, Wu J, Li P, Wang X, Zhu N, Wu P, Yang B (2012b). Experimental study of zero-valent iron induced nitrobenzene reduction in groundwater: The effects of pH, iron dosage, oxygen and common dissolved anions. Chemical Engineering Journal, 184: 198–204
Yu J, Kan Y, Rapp M, Danner E, Wei W, Das S, Miller D R, Chen Y, Waite J H, Israelachvili J N (2013). Adaptive hydrophobic and hydrophilic interactions of mussel foot proteins with organic thin films. Proceedings of the National Academy of Sciences of the United States of America, 110(39): 15680–15685
Zabihi Z, Araghi H (2016a). Effect of functional groups on thermal conductivity of graphene/paraffin nanocomposite. Physics Letters, 380(45): 3828–3831
Zabihi Z, Araghi H (2016b). Monte Carlo simulations of effective electrical conductivity of graphene/poly(methyl methacrylate) nanocomposite: Landauer-Buttiker approach. Synthetic Metals, 217: 87–93
Zhu H, Jia Y, Wu X, Wang H (2009). Removal of arsenic from water by supported nano zero-valent iron on activated carbon. Journal of Hazardous Materials, 172(2-3): 1591–1596
Acknowledgements
This research was supported by the National Nature Science Foundation of China (Grant Nos. 51368025 and 51068011).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Wang, X., Lian, W., Sun, X. et al. Immobilization of NZVI in polydopamine surface-modified biochar for adsorption and degradation of tetracycline in aqueous solution. Front. Environ. Sci. Eng. 12, 9 (2018). https://doi.org/10.1007/s11783-018-1066-3
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11783-018-1066-3