Abstract
Purpose
A randomized pilot trial evaluated the hypothesis that early intervention lessens sexual dysfunction in the first year on aromatase inhibitors. A secondary aim was comparing the efficacy of two vaginal moisturizers.
Methods
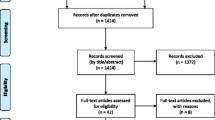
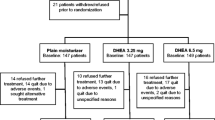
Fifty-seven postmenopausal women with early stage breast cancer starting aromatase inhibitors were randomized to three treatment groups. All received a handout on managing sexual and other side effects. The Usual Care group received no additional therapy. The Active Treatment groups received a 6-month supply of a vaginal moisturizer (hyaluronic acid-based in Active Group-H and prebiotic in Active Group-P) and a vaginal lubricant and dilator, plus access to an educational website and phone coaching. Questionnaires completed at baseline, 6, and 12 months included the Female Sexual Function Index (FSFI), Menopausal Sexual Interest Questionnaire (MSIQ), Female Sexual Distress Scale-Revised (FSDS-R), and a menopausal symptom scale.
Results
Forty-nine women (86%) provided follow-up data. Mean age was 59 and 77% were non-Hispanic Caucasian. Sexual function was impaired at baseline, but remained stable over 12 months for all groups. The combined active treatment group had less dyspareunia (P = 0.07) and sexual distress (P = 0.02) at 6 months than the Usual Care group. At 6 months, the Active-H group improved significantly more than the Active-P group on FSFI total score (P = 0.04).
Conclusions
Sexual counseling helped women maintain stable sexual function on aromatase inhibitors. Active intervention resulted in better outcomes at 6 months.
Implications for Cancer Survivors
This promising pilot trial suggests a need for more research on preventive counseling to maintain sexual function during aromatase inhibitor treatment.
Similar content being viewed by others
References
Early Breast Cancer Trialists’ Collaborative Group (EBCTCG), Dowsett M, Forbes JF, Bradley R, Ingle J, Aihara T, Bliss J, Boccardo F, Coates A, Coombes RC, Cuzick J, Dubsky P, Gnant M, Kaufmann M, Kilburn L, Perrone F, Rea D, Thürlimann B, van de Velde C, Pan H, Peto R, Davies C, Gray R. Aromatase inhibitors versus tamoxifen in early breast cancer; patient-level meta-analysis of the randomised trials. Lancet. 2015; 386:1341–1352.
Goss PE, Ingle JN, Pritchard KI, Robert NJ, Muss H, Gralow J, Gelmon K, Whelan T, Strasser-Weippl K, Rubin S, Sturtz K, Wolff AC, Winer E, Hudis C, Stopeck A, Beck JT, Kaur JS, Whelan K, Tu D, Parulekar WR. Extending aromatase-inhibitor adjuvant therapy to 10 years. N Eng J Med. 2016.
Bernhard J, Luo W, Ribi K, Colleoni M, Burstein HJ, Tondini C, Pinotti G, Spazzapan S, Ruhstaller T, Puglisi F, Pavesi L, Parmar V, Regan MM, Pagani O, Fleming GF, Francis PA, Price KN, Coates AS, Gelber RD, Goldhirsch A, Walley BA. Patient-reported outcomes with adjuvant exemestane versus tamoxifen in premenopausal women with early breast cancer undergoing ovarian suppression (TEXT and SOFT): a combined analysis of two phase 3 randomised trials. Lancet Oncol. 2015;16:848–58.
Burstein HJ, Lacchetti C, Anderson H, Buchholz TA, Davidson NE, Gelmon KE, Giordano SH, Hudis CA, Solky AJ, Stearns V, Winer EP, Griggs JJ. Adjuvant endocrine therapy for women with hormone receptor-positive breast cancer: American Society of Clinical Oncology Clinical Practice Guideline Update on ovarian suppression. J Clin Oncol. 2016;34:1689–701.
Baumgart J, Nilsson K, Evers AS, Kallak TK, Poromaa IS. Sexual dysfunction in women on adjuvant endocrine therapy after breast cancer. Menopause. 2013;20:162–8.
Baumgart J, Nilsson K, Stavreus Evers A, Kunovac Kallak T, Kushnir MM, Bergquist J, Sundström Poromaa I. Androgen levels during adjuvant endocrine therapy in postmenopausal breast cancer patients. Climacteric. 2014;17:48–54.
Cappelletti M, Wallen K. Increasing women’s sexual desire: the comparative effectiveness of estrogens and androgens. Horm Behav. 2016;78:178–93.
Schover LR, Baum GP, Fuson LA, Brewster A, Melhem-Bertrandt A. Sexual problems during the first 2 years of adjuvant treatment with aromatase inhibitors. J Sex Med. 2014;11:3102–11.
Groenvold M. Health-related quality of life in early breast cancer. Dan Med Bull. 2010;57:B4814.
Ganz PA, Petersen L, Bower JE, Crespi CM. Impact of adjuvant endocrine therapy on quality of life and symptoms: observational data over 12 months from the mind-body study. J Clin Oncol. 2016;34:816–24.
Hamidou Z, Dabakuyo TS, Bonnetain F. Impact of response shift on longitudinal quality-of-life assessment in cancer clinical trials. Expert Rev Pharmacoecon Outcomes Res. 2011;11:549–59.
Lutfey KE, Link CL, Rosen RC, Wiegel M, McKinlay JB. Prevalence and correlates of sexual activity and function in women: results from the Boston Area Community Health (BACH) survey. Arch Sex Behav. 2009;38:514–27.
Carter J, Stabile C, Seidel B, Baser RE, Gunn AR, Chi S, Steed RF, Goldfarb S, Goldfrank DJ. Baseline characteristics and concerns of female cancer patients/survivors seeking treatment at a female sexual medicine program. Support Care Cancer. 2015;23:2255–65.
Wiegel M, Meston C, Rosen R. The Female Sexual Function Index (FSFI): cross-validation and development of clinical cutoff scores. J Sex Marital Ther. 2005;31:1–20.
Baser RE, Li Y, Carter J. Psychometric validation of the Female Sexual Function Index (FSFI) in cancer survivors. Cancer. 2012;118:4606–18.
Carter J, Goldfrank D, Schover LR. Simple strategies for vaginal health promotion in cancer survivors. J Sex Med. 2011;8:549–59.
Schover LR, Yuan Y, Fellman BM, Odensky E, Lewis PE, Martinetti P. Efficacy trial of an internet-based intervention for cancer-related female sexual dysfunction. J Natl Compr Cancer Netw. 2013;11:1389–97.
Taves DR. The use of minimization in clinical trials. Contemp Clin Trials. 2010;31:180–4.
Rosen RC, Lobo RA, Block BA, Yang HM, Zipfel LM. Menopausal Sexual Interest Questionnaire (MSIQ): a unidimensional scale for the assessment of sexual interest in postmenopausal women. J Sex Marital Ther. 2004;30:235–50.
Derogatis L, Clayton A, Lewis-D’Agostino D, Wunderlich G, Fu Y. Validation of the female sexual distress scale-revised for assessing distress in women with hypoactive sexual desire disorder. J Sex Med. 2008;5:357–64.
Cella D, Land SR, Chang C-H, Day R, Costantino JP, Wolmark N, Ganz PA. Symptom measurement in the Breast Cancer Prevention Trial (BCPT) (P-1): psychometric properties of a new measure of symptoms for midlife women. Breast Cancer Res Treat. 2008;109:515–26.
Lindau ST, Hoffmann JN, Lundeen K, Jaszczak A, McClintock MK, Jordan JA. Vaginal self-swab specimen collection in a home-based survey of older women: methods and applications. J Gerontol Social Sciences. 2009;64B(S1):106–18.
Roy S, Caillouette JC, Faden JS, Roy T, Ramos DE. Improving appropriate use of antifungal medications: the role of an over-the-counter vaginal pH self-test device. Infect Dis Obstet Gynecol. 2003;11:209–16.
Chen J, Geng L, Song X, Li H, Giordan N, Liao Q. Evaluation of the efficacy and safety of hyaluronic acid vaginal gel to ease vaginal dryness: a multicenter, randomized, controlled, open-label, parallel-group, clinical trial. J Sex Med. 2013;10:1575–84.
Stute P. Is vaginal hyaluronic acid as effective as vaginal estriol for vaginal dryness relief? Arch Gynecol Obstet. 2013;288:1199–201.
Ekin M, Yasar L, Savan K, Temur M, Uhri M, Gencer I, Kıvanç E. The comparison of hyaluronic acid vaginal tablets with estradiol vaginal tablets in the treatment of atrophic vaginitis: a randomized controlled trial. Arch Gynecol Obstet. 2011;283:539–43.
Law E, Kelvin JF, Thom B, Riedel E, Tom A, Carter J, Alektiar KM, Goodman KA. Prospective study of vaginal dilator use adherence and efficacy following radiotherapy. Radiother Oncol. 2015;116:149–55.
Miles T, Johnson N. Vaginal dilator therapy for women receiving pelvic radiotherapy. Cochrane Database Syst Rev. 2014;8:CD007291.
Kallak TK, Baumgart J, Göransson E, Nilsson K, Poromaa IS, Stavreus-Evers A. Aromatase inhibitors affect vaginal proliferation and steroid hormone receptors. Menopause. 2014;21:383–90.
Kallak TK, Baumgart J, Nilsson K, Åkerud H, Poromaa IS, Stavreus-Evers A. Vaginal gene expression during treatment with aromatase inhibitors. Clin Breast Cancer. 2015;15:527–35.
Gandaglia G, Suardi N, Cucchiara V, Bianchi M, Shariat SF, Roupret M, Salonia A, Montorsi F, Briganti A. Penile rehabilitation after radical prostatectomy: does it work? Transl Androl Urol. 2015;4:110–23.
Meldrum DR, Burnett AL, Dorey G, Esposito K, Ignarro LJ. Erectile hydraulics: maximizing inflow while minimizing outflow. J Sex Med. 2014;11:1208–20.
Lin HC, Yang WL, Zhang JL, Dai YT, Wang R. Penile rehabilitation with a vacuum erectile device in an animal model is related to an antihypoxic mechanism: blood gas evidence. Asian J Androl. 2013;15:387–90.
Traish AM, Botchevar E, Kim NN. Biochemical factors modulating female genital sexual arousal physiology. J Sex Med. 2010;7:2925–46.
Le Ray I, Dell’Aniello S, Bonnetain F, Azoulay L, Suissa S. Local estrogen therapy and risk of breast cancer recurrence among hormone-treated patients: a nested case-control study. Breast Cancer Res Treat. 2012;135:603–9.
Committee Opinion No. 659. The use of vaginal estrogen in women with a history of estrogen-dependent breast cancer. Obstet Gynecol. 2016;127:393–396.
Donders G, Neven P, Moegele M, Lintermans A, Bellen G, Prasauskas V, Grob P, Ortmann O, Buchholz S. Ultra-low-dose estriol and lactobacillus acidophilus vaginal tablets (Gynoflor®) for vaginal atrophy in postmenopausal breast cancer patients on aromatase inhibitors: pharmacokinetic, safety, and efficacy phase I clinical study. Breast Cancer Res Treat. 2014;145:371–9.
Goetsch MF, Lim JY, Caughey AB. A practical solution for dyspareunia in breast cancer survivors: a randomized controlled trial. J Clin Oncol. 2015;33:3394–400.
Juraskova I, Jarvis S, Mok K, Peate M, Meiser B, Cheah BC, Mireskandari S, Friedlander M. The acceptability, feasibility, and efficacy (phase l/II study) of the OVERcome (olive oil, vaginal exercise, and moisturizer) intervention to improve dyspareunia and alleviate sexual problems in women with breast cancer. J Sex Med. 2013;20:2549–58.
Brown JM, Hess KL, Brown S, Murphy C, Waldman AL, Hezareh M. Intravaginal practices and risk of bacterial vaginosis and candidiasis infection among a cohort of women in the United States. Obstet Gynecol. 2013;121:773–80.
Mocellin S, Pilati P, Briarava M, Nitti D. Breast Cancer Chemoprevention: A Network Meta-Analysis of Randomized Controlled Trials. J Natl Cancer Inst. 2016;108.
Jennings S, Philip EJ, Nelson C, Schuler T, Starr T, Jandorf L, Temple L, Garcia E, Carter J, DuHamel K. Barriers to recruitment in psycho-oncology: unique challenges in conducting research focusing on sexual health in female survivorship. Psychooncology. 2014;23:1192–5.
Acknowledgments
This work was supported by a grant from the University of Texas MD Anderson Cancer Center, Duncan Family Institute for Cancer Prevention and Risk Assessment. Support was provided, in part, by the Patient-Reported Outcomes, Survey, and Population Research (PROSPR) Shared Resource through a Cancer Center Support Grant (CA16672, PI: R. DePinho, MD Anderson Cancer Center), from the National Cancer Institute, National Institutes of Health. Vaginal moisturizer for the study was donated by Fidia Pharma, USA, Inc., and by Laclede, Inc. The authors would like to thank Lisa Fuson, APN, for assistance in recruiting participants.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Pragati Advani, Abenaa Brewster, and George P. Baum declare that they have no conflict of interest. Leslie Schover acknowledges that Fidia Pharma, USA, Inc., and Laclede, Inc., donated vaginal moisturizer for the study. She also holds a financial interest in Will2Love, LLC, a digital health company based in part on the intervention in reference 17.
Ethical approval
All procedures performed in studies involving human participants were performed under an approved protocol and in accordance with the ethical standards of the institutional research committee of the University of Texas MD Anderson Cancer Center and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Additional information
Leslie R. Schover retired from University of Texas MD Anderson Cancer Center, Houston, TX, USA.
Rights and permissions
About this article
Cite this article
Advani, P., Brewster, A.M., Baum, G.P. et al. A pilot randomized trial to prevent sexual dysfunction in postmenopausal breast cancer survivors starting adjuvant aromatase inhibitor therapy. J Cancer Surviv 11, 477–485 (2017). https://doi.org/10.1007/s11764-017-0606-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11764-017-0606-3