Abstract
Objective
Although surgical ventricular restoration for ischemic cardiomyopathy is expected as an alternative or bridge to heart transplantation, post-operative remodeling of left ventricle (LV) needs to be addressed. This study aimed to examine the effect of basic fibroblast growth factor (bFGF), which induces angiogenesis and tissue regeneration in ischemic myocardium, to prevent remodeling after surgical ventricular restoration (SVR) using a rat ischemic cardiomyopathy model.
Methods
Four weeks after coronary artery ligation, rats were divided into two groups: rats treated with SVR alone (SVR; n = 21), and rats treated with SVR and local sustained release of bFGF using gelatin hydrogel sheet (SVR + bFGF; n = 22). Cardiac function was assessed by serial echocardiography and cardiac catheterization. Cardiac tissue sections were histologically examined for vascular density and fibrosis.
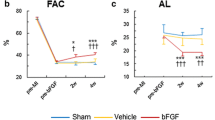
Results
Higher systolic function and lower LV end-diastolic pressure (LVEDP) were observed in rats treated with SVR + bFGF (SVR vs SVR + bFGF; Ees: 0.22 ± 0.11 vs 0.33 ± 0.22 mmHg/μL, p = 0.0328; LVEDP: 12.7 ± 7.0 vs 8.5 ± 4.3 mmHg, p = 0.0230). LV area tended to be lower in rats treated with SVR + bFGF compared to rats treated with SVR alone (left-ventricular end-diastolic area: 0.66 ± 0.07 vs 0.62 ± 0.07 cm2, p = 0.071). Vascular density tended to be higher in rats treated with SVR + bFGF than those without bFGF (23.3 ± 8.1 vs 28.8 ± 9.5/mm2, p = 0.0509).
Conclusions
BFGF induced angiogenesis and attenuated remodeling after SVR which secured the efficacy of SVR in a rat ischemic cardiomyopathy model.




Similar content being viewed by others
References
Adhyapak SM, Menon PG, Parachuri VR, Shetty DP, Fantini F. Characterization of dysfunctional remote myocardium in left ventricular anterior aneurysms and improvements following surgical ventricular restoration using cardiac magnetic resonance imaging: preliminary results. Interact Cardiovasc Thorac Surg. 2014;19:368–74.
Matsui Y, Fukada Y, Naito Y, Sasaki S. Integrated overlapping ventriculoplasty combined with papillary muscle plication for severely dilated heart failure. J Thorac Cardiovasc Surg. 2004;127:1221–3.
Suma H, Isomura T, Horii T, Sato T, Kikuchi N, Iwahashi K, et al. Nontransplant cardiac surgery for end-stage cardiomyopathy. J Thorac Cardiovasc Surg. 2000;119:1233–44.
Dor V, Sabatier M, Di Donato M, Montiglio F, Toso A, Maioli M. Efficacy of endoventricular patch plasty in large postinfarction akinetic scar and severe left ventricular dysfunction: comparison with a series of large dyskinetic scars. J Thorac Cardiovasc Surg. 1998;116:50–9.
Isomura T, Horii T, Suma H, Buckberg GD, Group R. Septal anterior ventricular exclusion operation (Pacopexy) for ischemic dilated cardiomyopathy: treat form not disease. Eur J Cardiothorac Surg. 2006;29(Suppl 1):S245–50.
Nishina T, Nishimura K, Yuasa S, Miwa S, Nomoto T, Sakakibara Y, et al. Initial effects of the left ventricular repair by plication may not last long in a rat ischemic cardiomyopathy model. Circulation. 2001;104:241–5.
Nomoto T, Nishina T, Tsuneyoshi H, Miwa S, Nishimura K, Komeda M. Effects of two inhibitors of renin-angiotensin system on attenuation of postoperative remodeling after left ventricular aneurysm repair in rats. J Card Surg. 2003;18(Suppl 2):S61–S6868.
Nomoto T, Nishina T, Miwa S, Tsuneyoshi H, Maruyama I, Nishimura K, et al. Angiotensin-converting enzyme inhibitor helps prevent late remodeling after left ventricular aneurysm repair in rats. Circulation. 2002;106:I115–9.
Tsuneyoshi H, Nishina T, Nomoto T, Kanemitsu H, Kawakami R, Unimonh O, et al. Atrial natriuretic peptide helps prevent late remodeling after left ventricular aneurysm repair. Circulation. 2004;110:174–9.
Carmeliet P. Mechanisms of angiogenesis and arteriogenesis. Nat Med. 2000;6:389–95.
Deindl E, Hoefer IE, Fernandez B, Barancik M, Heil M, Strniskova M, et al. Involvement of the fibroblast growth factor system in adaptive and chemokine-induced arteriogenesis. Circ Res. 2003;92:561–8.
Iwakura A, Tabata Y, Miyao M, Ozeki M, Tamura N, Ikai A, et al. Novel method to enhance sternal healing after harvesting bilateral internal thoracic arteries with use of basic fibroblast growth factor. Circulation. 2000;102:307–11.
Iwakura A, Tabata Y, Tamura N, Doi K, Nishimura K, Nakamura T, et al. Gelatin sheet incorporating basic fibroblast growth factor enhances healing of devascularized sternum in diabetic rats. Circulation. 2001;104:325-329.
Nakajima H, Sakakibara Y, Tambara K, Iwakura A, Doi K, Marui A, et al. Therapeutic angiogenesis by the controlled release of basic fibroblast growth factor for ischemic limb and heart injury: toward safety and minimal invasiveness. J Artif Org. 2004;7:58−61.
Kumagai M, Marui A, Tabata Y, Takeda T, Yamamoto M, Yonezawa A, et al. Safety and efficacy of sustained release of basic fibroblast growth factor using gelatin hydrogel in patients with critical limb ischemia. Heart Vessels. 2016;31:713–21.
Kumagai M, Minakata K, Masumoto H, Yamamoto M, Yonezawa A, Ikeda T, et al. A therapeutic angiogenesis of sustained release of basic fibroblast growth factor using biodegradable gelatin hydrogel sheets in a canine chronic myocardial infarction model. Heart Vessels. 2018;33(10):1251–7.
Li Z, Masumoto H, Jo JI, Yamazaki K, Ikeda T, Tabata Y, et al. Sustained release of basic fibroblast growth factor using gelatin hydrogel improved left ventricular function through the alteration of collagen subtype in a rat chronic myocardial infarction model. Gen Thorac Cardiovasc Surg. 2018;66(11):641–7.
Yamamoto M, Ikada Y, Tabata Y. Controlled release of growth factors based on biodegradation of gelatin hydrogel. J Biomater Sci Polym Ed. 2001;12:77–88.
Masumoto H, Matsuo T, Yamamizu K, Uosaki H, Narazaki G, Katayama S, et al. Pluripotent stem cell-engineered cell sheets reassembled with defined cardiovascular populations ameliorate reduction in infarct heart function through cardiomyocyte-mediated neovascularization. Stem Cells. 2012;30:1196–205.
Dor V, Sabatier M, Di Donato M, Maioli M, Toso A, Montiglio F. Late hemodynamic results after left ventricular patch repair associated with coronary grafting in patients with postinfarction akinetic or dyskinetic aneurysm of the left ventricle. J Thorac Cardiovasc Surg. 1995;110:1291–9; discussion 1300–1.
Carmichael BB, Setser RM, Stillman AE, Lieber ML, Smedira NG, McCarthy PM, et al. Effects of surgical ventricular restoration on left ventricular function: dynamic MR imaging. Radiology. 2006;241:710–7.
Di Donato M, Sabatier M, Dor V, Gensini GF, Toso A, Maioli M, et al. Effects of the Dor procedure on left ventricular dimension and shape and geometric correlates of mitral regurgitation one year after surgery. J Thorac Cardiovasc Surg. 2001;121:91–6.
Ascione R, Lim KH, Chamberlain M, Al-Ruzzeh S, Angelini GD. Early and late results of partial left ventriculectomy: single center experience and review of the literature. J Card Surg. 2003;18:190–6.
Hwang HY, Kim JS, Cho KR, Kim KB. Surgical anterior ventricular endocardial restoration performed with total arterial revascularization: serial 5-year follow-up. J Thorac Cardiovasc Surg. 2014;148:529–35.
O'Neill JO, Starling RC, McCarthy PM, Albert NM, Lytle BW, Navia J, et al. The impact of left ventricular reconstruction on survival in patients with ischemic cardiomyopathy. Eur J Cardiothorac Surg. 2006;30:753–9.
French BA, Kramer CM. Mechanisms of post-infarct left ventricular remodeling. Drug Discov Today Dis Mech. 2007;4:185–96.
Kramer CM, Lima JA, Reichek N, Ferrari VA, Llaneras MR, Palmon LC, et al. Regional differences in function within noninfarcted myocardium during left ventricular remodeling. Circulation. 1993;88:1279–88.
Jackson BM, Gorman JH, Moainie SL, Guy TS, Narula N, Narula J, et al. Extension of borderzone myocardium in postinfarction dilated cardiomyopathy. J Am Coll Cardiol. 2002;40:1160–7; discussion 1168–71.
Heusch G, Schulz R, Rahimtoola SH. Myocardial hibernation: a delicate balance. Am J Physiol Heart Circ Physiol. 2005;288:H984–99.
Maes A, Flameng W, Nuyts J, Borgers M, Shivalkar B, Ausma J, et al. Histological alterations in chronically hypoperfused myocardium. Correlation with PET findings. Circulation. 1994;90:735–45.
Cornel JH, Bax JJ, Elhendy A, Maat AP, Kimman GJ, Geleijnse ML, et al. Biphasic response to dobutamine predicts improvement of global left ventricular function after surgical revascularization in patients with stable coronary artery disease: implications of time course of recovery on diagnostic accuracy. J Am Coll Cardiol. 1998;31:1002–100.
Acknowledgments
This work was supported by Grants-in-Aid for Scientific Research from the Ministry of Education, Science, Sports, and Culture of Japan (to T.I.) [Grant Number 22591540]. We are grateful to Mrs. F. Kataoka (Kyoto Univ.) for assistances in histological studies, and to Dr. A. Sugimoto (Kyoto Univ.) for assistance with surgical procedures and examinations such as echocardiography and cardiac catheterization. We thank Dr. Hemant Poudyal (Kyoto Univ.) for critical reading of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared that no conflict of interest exists.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nagasawa, A., Masumoto, H., Yanagi, S. et al. Basic fibroblast growth factor attenuates left-ventricular remodeling following surgical ventricular restoration in a rat ischemic cardiomyopathy model. Gen Thorac Cardiovasc Surg 68, 311–318 (2020). https://doi.org/10.1007/s11748-019-01187-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11748-019-01187-3