Abstract
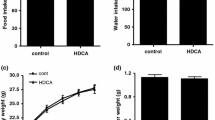
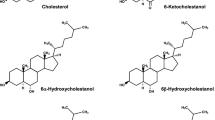
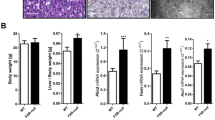
The farnesoid X receptor (FXR) is a major nuclear receptor of bile acids; its activation suppresses sterol regulatory element-binding protein 1c (SREBP1c)-mediated lipogenesis and decreases the lipid contents in the liver. There are many reports showing that the administration of ursodeoxycholic acid (UDCA) suppresses lipogenesis and reduces the lipid contents in the liver of experimental animals. Since UDCA is not recognized as an FXR agonist, these effects of UDCA cannot be readily explained by its direct activation of FXR. We observed that the dietary administration of UDCA in mice decreased the expression levels of SREBP1c and its target lipogenic genes. Alpha- and β-muricholic acids (MCA) and cholic acid (CA) were the major bile acids in the mouse liver but their contents decreased upon UDCA administration. The hepatic contents of chenodeoxycholic acid and deoxycholic acid (DCA) were relatively low but were not changed by UDCA. UDCA did not show FXR agonistic or antagonistic potency in in vitro FXR transactivation assay. Taking these together, we deduced that the above-mentioned change in hepatic bile acid composition induced upon UDCA administration might cause the relative increase in the FXR activity in the liver, mainly by the reduction in the content of β-MCA, a farnesoid X receptor antagonist, which suggests a mechanism by which UDCA suppresses lipogenesis and decreases the lipid contents in the mouse liver.




Similar content being viewed by others
Abbreviations
- ACC:
-
Acetyl-CoA carboxylase
- CA:
-
Cholic acid
- CDCA:
-
Chenodeoxycholic acid
- CE:
-
Cholesteryl ester
- DCA:
-
Deoxycholic acid
- DGAT:
-
Diacylglycerol acyltransferase
- DMEM:
-
Dulbecco’s minimum essential medium
- CYP:
-
Cytochrome P450
- FXR:
-
Farnesoid X receptor
- HDCA:
-
Hyodeoxycholic acid
- FAS:
-
Fatty acid synthase
- LCーMS:
-
Liquid chromatographyーmass spectrometry
- LXR:
-
Liver X receptor
- MCA:
-
Muricholic acid
- MUFA:
-
Monounsaturated fatty acid
- PL:
-
Phospholipid
- RXR:
-
Retinoid X receptor
- SHP:
-
Small heterodimer partner
- SCD:
-
Stearoyl-CoA desaturase
- SMILE:
-
Small heterodimer partner-interacting leucine zipper protein
- SREBP1c:
-
Sterol regulatory element-binding protein 1c
- TG:
-
Triglyceride
- UDCA:
-
Ursodeoxycholic acid
References
Makishima M, Okamoto AY, Repa JJ, Tu H, Learned RM, Luk A, Hull MV, Lustig KD, Mangelsdorf DJ, Shan B (1999) Identification of a nuclear receptor for bile acids. Science 284(5418):1362–1365
Parks DJ, Blanchard SG, Bledsoe RK, Chandra G, Consler TG, Kliewer SA, Stimmel JB, Willson TM, Zavacki AM, Moore DD, Lehmann JM (1999) Bile acids: natural ligands for an orphan nuclear receptor. Science 284(5418):1365–1368
Li T, Chiang JY (2014) Bile acid signaling in metabolic disease and drug therapy. Pharmacol Rev 66:948–983
Kuipers F, Bloks VW, Groen AK (2014) Beyond intestinal soap—bile acids in metabolic control. Nat Rev Endocrinol 10:488–498
Chawla A, Repa JJ, Evans RM, Mangelsdorf DJ (2001) Nuclear receptors and lipid physiology: opening the X-files. Science 294:1866–1870
Hoeke MO, Heegsma J, Hoekstra M, Moshage H, Faber KN (2014) Human FXR regulates SHP expression through direct binding to an LRH-1 binding site, independent of an IR-1 and LRH-1. PLoS One 9:e88011
Watanabe M, Houten SM, Wang L, Moschetta A, Mangelsdorf DJ, Heyman RA, Moore DD, Auwerx J (2004) Bile acids lower triglyceride levels via a pathway involving FXR, SHP, and SREBP-1c. J Clin Invest 113:1408–1418
Shen LL, Liu H, Peng J, Gan L, Lu L, Zhang Q, Li L, He F, Jiang Y (2011) Effects of farnesoid X receptor on the expression of the fatty acid synthetase and hepatic lipase. Mol Biol Rep 38:553–559
Bilz S, Samuel V, Morino K, Savage D, Choi CS, Shulman GI (2006) Activation of the farnesoid X receptor improves lipid metabolism in combined hyperlipidemic hamsters. Am J Physiol Endocrinol Metab 290:E716–E722
Buko VU, Kuzmitskaya-Nikolaeva IA, Naruta EE, Lukivskaya OY, Kirko SN, Tauschel HD (2011) Ursodeoxycholic acid dose-dependently improves liver injury in rats fed a methionine- and choline-deficient diet. Hepatol Res 41:647–659
Mahmoud AA, Elshazly SM (2014) Ursodeoxycholic acid ameliorates fructose-induced metabolic syndrome in rats. PLoS One 9:e106993
Oh AR, Bae JS, Lee J, Shin E, Oh BC, Park SC, Cha JY (2016) Ursodeoxycholic acid decreases age-related adiposity and inflammation in mice. BMB Rep 49:105–110
Quintero P, Pizarro M, Solís N, Arab JP, Padilla O, Riquelme A, Arrese M (2014) Bile acid supplementation improves established liver steatosis in obese mice independently of glucagon-like peptide-1 secretion. J Physiol Biochem 70:667–674
Tsuchida T, Shiraishi M, Ohta T, Sakai K, Ishii S (2012) Ursodeoxycholic acid improves insulin sensitivity and hepatic steatosis by inducing the excretion of hepatic lipids in high-fat diet-fed KK-Ay mice. Metabolism 61:944–953
Mueller M, Thorell A, Claudel T, Jha P, Koefeler H, Lackner C, Hoesel B, Fauler G, Stojakovic T, Einarsson C, Marschall HU, Trauner M (2015) Ursodeoxycholic acid exerts farnesoid X receptor-antagonistic effects on bile acid and lipid metabolism in morbid obesity. J Hepatol 62:1398–1404
Watanabe S, Fujita K (2014) Dietary hyodeoxycholic acid exerts hypolipidemic effects by reducing farnesoid X receptor antagonist bile acids in mouse enterohepatic tissues. Lipids 49:963–973
Makishima M, Lu TT, Xie W, Whitfield GK, Domoto H, Evans RM, Haussler MR, Mangelsdorf DJ (2002) Vitamin D receptor as an intestinal bile acid sensor. Science 296(5571):1313–1316
Hu X, Bonde Y, Eggertsen G, Rudling M (2014) Muricholic bile acids are potent regulators of bile acid synthesis via a positive feedback mechanism. J Intern Med 275:27–38
Sayin SI, Wahlström A, Felin J, Jäntti S, Marschall HU, Bamberg K, Angelin B, Hyötyläinen T, Orešič M, Bäckhed F (2013) Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-beta-muricholic acid, a naturally occurring FXR antagonist. Cell Metab 17:225–235
Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37:911–917
Kunishima M, Kawachi C, Hioki K, Terao K, Tani S (2001) Formation of carboxamides by direct condensation of carboxylic acids and amines in alcohols using a new alcohol- and water-soluble condensing agent: DMT-MM. Tetrahedron 57:1551–1558
Horton JD, Shah NA, Warrington JA, Anderson NN, Park SW, Brown MS, Goldstein JL (2003) Combined analysis of oligonucleotide microarray data from transgenic and knockout mice identifies direct SREBP target genes. Proc Natl Acad Sci USA 100:12027–12032
De Leon MP, Carulli N, Loria P, Iori R, Zironi (1980) Cholesterol absorption during bile acid feeding. Effect of ursodeoxycholic acid (UDCA) administration. Gastroenterol 78:214–219
Uchida K, Akiyoshi T, Igimi H, Takase H, Nomura Y, Ishihara S (1991) Differential effects of ursodeoxycholic acid and ursocholic acid on the formation of biliary cholesterol crustals in mice. Lipids 26:526–530
Serhan CN, Yacoubian S, Yang R (2008) Anti-inflammatory and pro-resolving lipid mediators. Annu Rev Pathol 3:279–312
Lew JL, Zhao A, Yu J, Huang L, De Pedro N, Peláez F, Wright SD, Cui J (2004) The farnesoid X receptor controls gene expression in a ligand- and promoter-selective fashion. J Biol Chem 279:8856–8861
Jiang C, Xie C, Lv Y, Li J, Krausz KW, Shi J, Brocker CN, Desai D, Amin SG, Bisson WH, Liu Y, Gavrilova O, Patterson AD, Gonzalez FJ (2015) Intestine-selective farnesoid X receptor inhibition improves obesity-related metabolic dysfunction. Nat Commun 6:10166. doi:10.1038/ncomms10166
Nie Y-F, Hu J, Yan X-H (2015) Cross-talk between bile acids and intestinal microbiota in host metabolism and health. J Zhejiang Univ Sci B 16:436–446
Li T, Matozel M, Boehme S, Kong B, Nilsson LM, Guo G, Ellis E, Chiang JYL (2011) Overexpression of cholesterol 7α-hydroxylase promotes hepatic bile acid synthesis and secretion and maintains cholesterol homeostasis. Hepatology 53:996–1006
Fedorowski T, Salen G, Calallilo A, Tint GS, Mosbach EH, Hall JC (1977) Metabolism of ursodeoxycholic acid in man. Gastroenterology 73:1131–1137
García-Cañaveras JC, Donato MT, Castell JV, Lahoz A (2012) Targeted profiling of circulating and hepatic bile acids in human, mouse, and rat using a UPLC-MRM-MS-validated method. J Lipid Res 53:2231–2241
Lee JM, Gang GT, Kim DK, Kim YD, Koo SH, Lee CH, Choi HS (2014) Ursodeoxycholic acid inhibits liver X receptor α-mediated hepatic lipogenesis via induction of the nuclear corepressor SMILE. J Biol Chem 289:1079–1091
Acknowledgements
This work was supported in part by a Grant-in-Aid for the 2015 Co-operative Research Project (Ippan Kenkyu) from the Cooperative Research Project from the Joint Usage/Research Center (Joint Usage/Research Center for Science-Based Natural Medicine), Institute of Natural Medicine, University of Toyama in 2015 (to Y. I. and K. F.) and by Grants-in-Aid from the Ministry of Education, Culture, Sports, Science and Technology of Japan (to S.W.) (Research Project Numbers: 23590873 and 26460903).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
We have no conflict of interest.
About this article
Cite this article
Fujita, K., Iguchi, Y., Une, M. et al. Ursodeoxycholic Acid Suppresses Lipogenesis in Mouse Liver: Possible Role of the Decrease in β-Muricholic Acid, a Farnesoid X Receptor Antagonist. Lipids 52, 335–344 (2017). https://doi.org/10.1007/s11745-017-4242-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11745-017-4242-5