Abstract
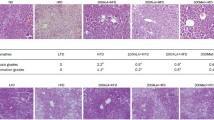
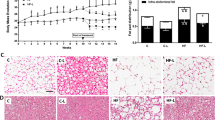
Lipogenesis is the process by which fatty acids are synthesized. In metabolic syndrome, an insulin resistant state along with high plasma levels of free fatty acids (FFA) and hyperglycemia may contribute to the lipogenic process. The aim of the present study was to investigate the effects of oral administration of metformin on the expression of lipogenic genes and glycemic profile in mice fed with low-carbohydrate high-fat diet by evaluating their metabolic profile. SWISS male mice were divided into 4 groups (N = 7) that were fed with standard (ST), standard plus metformin (ST + MET), low-carbohydrate high-fat diet (LCHFD) and low-carbohydrate high-fat diet plus metformin (LCHFD + MET) (100 mg kg−1 diet) diets respectively. Food intake, body weight and blood parameters, such as glucose tolerance, insulin sensitivity, glucose, HDL-c, total cholesterol, triglycerides, ASL and ALT levels were assessed. Histological analyses were performed on hematoxylin and eosin-stained epididymal adipose tissue histological specimens. The expression levels of peroxisome proliferator-activated receptor (PPARγ), sterol regulatory element-binding protein 1 (SREBP1), fatty acid synthase (FAS) and acetyl-CoA carboxylase (ACC), were assessed by RT-PCR. This study showed that metformin decreased adipocyte area, body weight and food consumption in obese animals when compared to the standard group. Furthermore, the expression of lipogenic markers in adipose tissue were diminished in obese animals treated with metformin. This data showed that oral administration of metformin improved glucose and lipid metabolic parameters in white adipose tissue by reducing the expression of lipogenesis markers, suggesting an important clinical application of MET in treating obesity-related diseases in metabolic syndrome.




Similar content being viewed by others
Abbreviations
- ACC:
-
Acetyl CoA carboxylase
- AMPK:
-
AMP-activated protein kinase
- FAS:
-
Fatty acid synthase
- FFA:
-
Free fat acids
- GAPDH:
-
Glyceraldehyde 3- phosphate dehydrogenase
- HDL:
-
High-density lipoprotein
- LCHFD:
-
Low-carbohydrate high-fat diet
- LCHFD + MET:
-
Low-carbohydrate high-fat diet plus metformin
- H&E:
-
Hematoxylin and eosin
- LDL:
-
Low-density lipoprotein
- MetS:
-
Metabolic syndrome
- PPARγ:
-
Peroxisome proliferator-activated receptor
- qRT-PCR:
-
Real time PCR
- SREBP-1:
-
Protein-1 sterol regulatory element binding
- ST:
-
Standard diet
- VLDL:
-
Very low-density lipoprotein
References
Alberti KG, Zimmet P, Shaw J (2005) The metabolic syndrome—a new worldwide definition. Lancet 366:1059–1062
Robert HE, Scott MG, Paul ZZ (2005) The metabolic syndrome. Lancet 365:1415–1428
Bjorntorp P (1991) Metabolic implications of body fat distribution. Diabetes Care 14:1132–1143
Tchernof A, Després J (2013) Pathophysiology of human visceral obesity: an update. Physiol Rev 93:359–404
Flegal KM et al (2010) Prevalence and trends in obesity among US adults, 1999–2008. JAMA 303:235–241
Bae NK, Kwon IS, Cho YC (2009) Ten year change of body mass index in Korean: 1997–2007. Korean J Obes 18:24–30
Mokdad AH et al (2003) Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. JAMA 289:76–79
Grundy SM (2004) Obesity, metabolic syndrome, and cardiovascular disease. J Clin Endocrinol Metab 89:2595–2600
Trayhurn P (2013) Hipoxia and adipose tissue function and dysfunction in obesity. Physiol Rev 93:1–21
Libby G et al (2009) New users of metformin are at low risk of incident cancer: a cohort study among people with type 2 diabetes. Diabetes Care 32:1620–1625
Taubes G (2009) Insulin resistance. Prosperity’s plague. Science 325:256–260
Giovannucci E et al (2010) Diabetes and cancer: a consensus report. Diabetes Care 33:1674–1685
He L et al (2009) Metformin and insulin suppress hepatic gluconeogenesis through phosphorylation of CREB binding protein. Cell 137:635–646
Correia S et al (2008) Mechanisms of action of metformin in type 2 diabetes and associated complications: an overview. Mini Rev Med Chem 8:1343–1354
Pollak MN (2012) Investigating metformin for cancer prevention and treatment: the end of the beginning. Cancer Discov 9:778–790
Viollet B et al (2012) Cellular and molecular mechanisms of metformin: an overview. Clin Sci 122:253–270
Girard J, Foifelle F, Ferré P (1997) Mechanisms by which carbohydrates regulate expression of genes for glycolytic and lipogenic enzymes. Annu Rev Nutr 17:325
Calder PC (2012) Mechanisms of action of (n-3) fatty acids. J Nutr 142:592–599
Puglisi MJ, Hasty AH, Saraswathi V (2011) The role of adipose tissue in mediating the beneficial effects of dietary fish oil. J Nutr Biochem 22:101–108
Lima TM et al (2007) Mechanisms by which fatty acids regulate leucocyte function. Clin Sci 113:65–77
Suchankova G et al (2005) Dietary polyunsaturated fatty acids enhance hepatic AMP-activated protein kinase activity in rats. Biochem Biophys Res Commun 326:851–858
Santos SHS et al (2013) Oral Angiotensin-(1-7) prevented obesity and hepatic inflammation by inhibition of resistin/TLR4/MAPK/NF-kappaB in rats fed with high-fat diet. Peptides 46:47–52
Andrade JMO et al (2014) Resveratrol increases brown adipose tissue thermogenesis markers by increasing SIRT1 and energy expenditure and decreasing fat accumulation in adipose tissue of mice fed a standard diet. Eur J Nutr 53:1503–1510
Montalvo AM (2012) Metformin improves healthspan and lifespan in mice. Nat Commun 4:2192
Santos SHS et al (2012) Increased circulating angiotensin-(1-7) protects white adipose tissue against development of a proinflammatory state stimulated by a high-fat diet. Regul Pept 178:64–70
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−∆∆CT method. Methods 25:402–408
Li Y et al (2011) AMPK phosphorylates and inhibits SREBP activity to attenuate hepatic steatosis and atherosclerosis in diet-induced insulin-resistant mice. Cell Metab 13:376–388
Liang G et al (2002) Diminished hepatic response to fasting/refeeding and liver X receptor agonists in mice with selective deficiency of sterol regulatory element-binding protein-1c. J Biol Chem 277:9520–9528
Desilets AR, Dhakal-Karki S, Dunican KC (2008) Role of metformin for weight management in patients without type 2 diabetes. Ann Pharmacother 42:817–826
Yukari M et al (2010) Metformin reduces body weight gain and improves glucose intolerance in high-fat diet-fed C57BL/6J mice. Biol Pharm Bull 33:963–970
Kuipers RS et al (2011) Saturated fat, carbohydrates and cardiovascular disease. Neth J Med 69:372–378
Augusto CSJ, Michelle RU, Ana TS (2008) Meformina e AMPK: um antigo fármaco e uma nova enzima no contexto da síndrome metabólica. Arquivos Brasileiros de Endocrinologia e Metabolismo 52:120–125
SBD-Sociedade Brasileira de Diabetes (2003) Consenso Brasileiro sobre Diabetes: Diagnóstico e classificação do Diabetes Mellitus e tratamento do Diabetes Mellitus Tipo 2. Diagraphic, Rio de Janeiro
Grosskopf I et al (1997) Metformin enhances clearance of chylomicrons and chylomicron remnants in nondiabetic mildly overweight glucose-intolerant subjects. Diabetes Care 20:1598–1602
Storlien LH et al (1991) Influence of dietary fat composition on development of insulin resistance in rats: relationship to muscle triglyceride and ω-3 fatty acids in muscle phospholipid. Diabetes 40(2):280–289
Cheng JT et al (2001) Metformin-like effects of Quei Fu Di Huang Wan, a Chinese herbal mixture, on streptozotocin-induced diabetic rat. Hormone Metab Res 33:727–732
Tang T, Reed MJ (2001) Exercise adds to metformin and acarbose efficacy in db/db mice. Metabolism 50:1049–1053
Souza CT et al (2003) Insulin secretion in monosodium glutamate (MSG) obese rats submitted to aerobic exercise training. Physiol Chem Phys Med 35:43–53
Santos RF et al (1995) Changes in insulin receptor tyrosine kinase activity associated with metformin treatment of type 2 diabetes. Diabetes Metabol 21:274–280
Elhayany A, Lustman A, Abel R, Attal-Singer J, Vinker S (2010) A low carbohydrate Mediterranean diet improves cardiovascular risk factors and diabetes control among overweight patients with type 2 diabetes mellitus: a 1-year prospective randomized intervention study. Diabetes Obes Metab 12:204–209
Alison BE (2013) Nutrition therapy recommendations for the management of adults with diabetes. Diabetes Care 36:3821–3842
Marshall JA, Hamman RF, Baxter J (1991) High-fat, low-carbohydrate diet and the etiology of non-insulin-dependent diabetes mellitus: the San Luis Valley Diabetes Study. Am J Epidemiol 134:590–603
Lamont BJ, Waters MF, Andrikopoulos S (2016) A low-carbohydrate high-fat diet increases weight gain and does not improve glucose tolerance, insulin secretion or β-cell mass in NZO mice. Nutr Diabetes 6:e194
Demetrius L (2005) Of mice and men. EMBO Rep 6:S39–S44
Kummitha CM, Kalhan SC, Saidel GM, Lai N (2014) Relating tissue/organ energy expenditure to metabolic fluxes in mouse and human: experimental data integrated with mathematical modeling. Physiol Rep 2:e12159
Feltenberger JD et al (2013) Oral formulation of angiotensin-(1-7) improves lipid metabolism and prevents high-fat diet-induced hepatic steatosis and inflammation in mice. Hypertension 62:324–330
Crespo TS et al (2016) Effects of omentectomy in addition to sleeve gastrectomy on the metabolic and inflammatory profiles of obese rats. Surg Obes Relat Dis 16:1–8
Andrade JMO et al (2014) Cross talk between angiotensin-(1-7)/mas axis and sirtuins in adipose tissue and metabolism of high-fat feed mice. Peptides 55:158–165
Andrade JMO et al (2014) Proteomic white adipose tissue analysis of obese mice fed with a high-fat diet and treated with oral angiotensin-(1-7). Peptides 60:56–62
Rosen ED et al (2002) C/EBPα induces adipogenesis through PPARγ: a unified pathway. Genes Dev 16:22–26
Shimomura I, Hammer RE, Ikemoto S, Brown MS, Goldstein JL (1999) Leptin reverses insulin resistance and diabetes mellitus in mice with congenital lipodystrophy. Nature 401:73–76
Shimomura I et al (2000) Decreased IRS-2 and increased SREBP-1c lead to mixed insulin resistance and sensitivity in livers of lipodystrophic and ob/ob mice. Mol Cell 6:77–86
Financial support
This work was partially supported by the Coordenadoria de Aperfeiçoamento do Pessoal de Nível Superior (CAPES) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Fundação de Amparo a Pesquisa do Estado de Minas Gerais (FAPEMIG).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
About this article
Cite this article
de Oliveira Santana, K.N., Lelis, D.F., Mendes, K.L. et al. Metformin Reduces Lipogenesis Markers in Obese Mice Fed a Low-Carbohydrate and High-Fat Diet. Lipids 51, 1375–1384 (2016). https://doi.org/10.1007/s11745-016-4209-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11745-016-4209-y